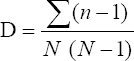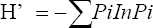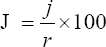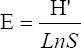Fungi diversity assessment under different tree species of Shere Hills soils in Jos North Local Government Area Plateau State, North Nigeria
A. S. Popoola, M. S. Chomini*, N. Janfa, M. J. Francis, C. Kambai, T. A. Erhabor, Y. E. Sadiku, J. A. Imoh, C. J. Ukanyirioha, M. Ukwadi, A. M. Kabir
Department of Forestry Technology, Federal College of Forestry Jos P.M.B 2019, lateau State, Nigeria
ABSTRACT
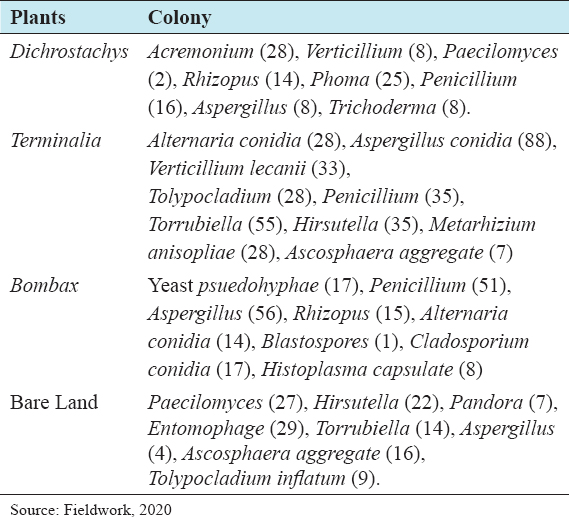
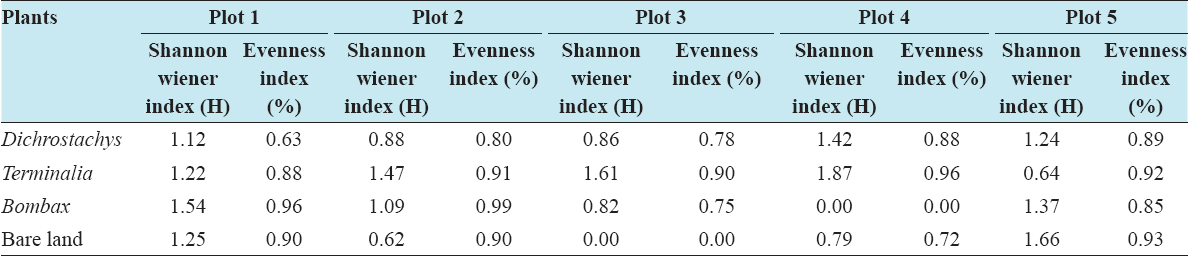
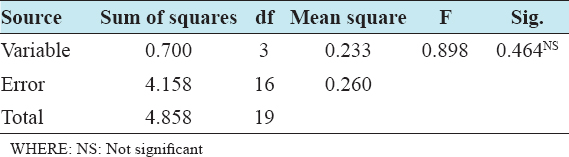
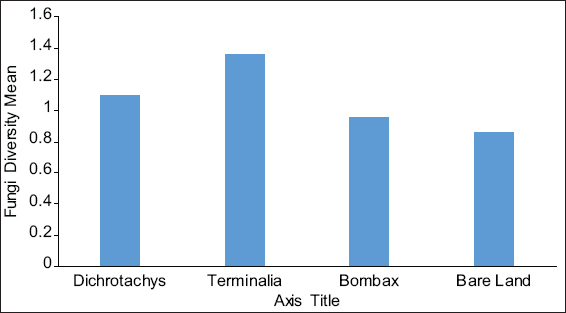
This study assessed the fungi composition under three different forest tree species cover and bare land in Shere Hills Jos, North Nigeria. Plants species considered are Dichrostachys, Terminalia, and Bombax. Two meters by 2 m plot under forest cover and adjacent open area were set up. Fungi species diversity indices (Shannon-Wiener index and species evenness) were calculated. Results revealed that there were 49 species distributed into 28 families (293 individuals) under the tree cover, while there were 53 species distributed into 33 families (451 individuals) in the open vegetation. The Jaccard index of similarity (79.49%) was high between the study sites. The result of this study showed that forest cover has a beneficial effect on soil properties and nutrient pool. Plants species richness and microbes population were also higher under the forest cover than in the open area. Species diversity and richness, soil nutrient, and microbial population were greatly influenced by forest cover.
Keywords: Soils, fungi, Shere Hills, diversity, tree species, plate count