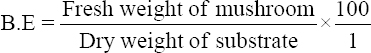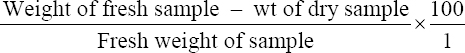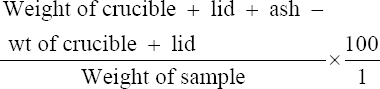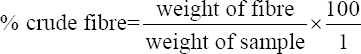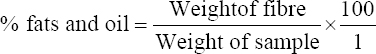Assessment of yield and nutritional composition of Pleurotus ostreatus (Jacq.Ex.Fr) p. Kumm. fruit bodies cultivated on dactylis glomerata L. and chloris gayana kunth
Okwulehie Ikechukwu Cyriacus1*, Aruoma Chukwunomso Anthony2, Ezeh Chidinma Godspower3
1Department of Plant Science and biotechnology, Michael Okpara University of Agriculture, Umudike, Abia State, 2Department of Plant Science and biotechnology, Michael Okpara University of Agriculture, Umudike, Abia State, 3Department of Biology, School of Biological Sciences, Federal University of Technology, Owerri, Imo State
ABSTRACT
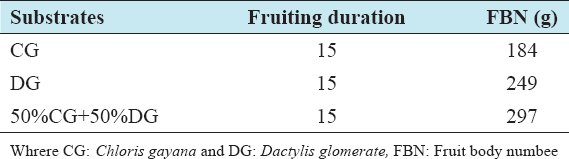
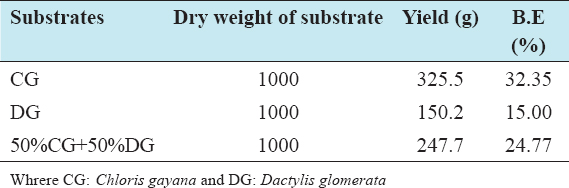
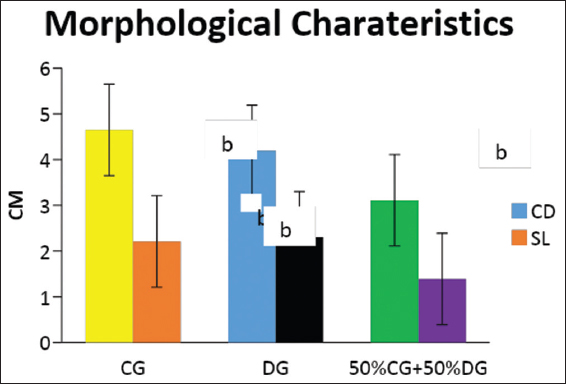
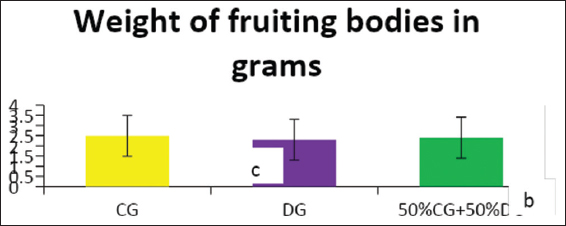
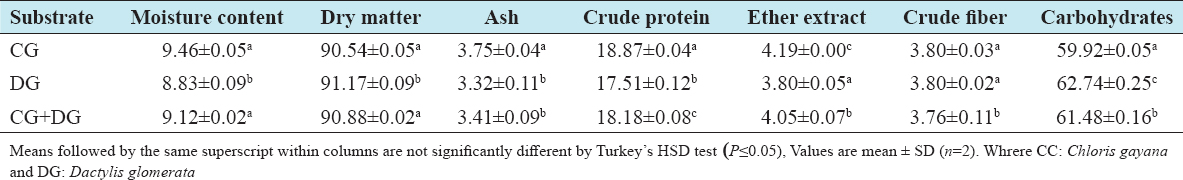
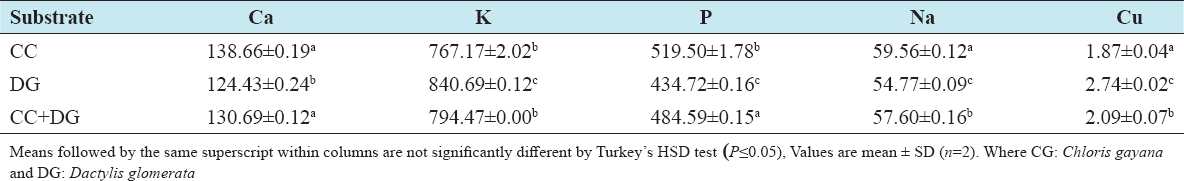
This study was conducted to determine the influence of agro-waste of Dactylis glomerata (DG) and Chloris gayana (CG) on the fructification, nutritional composition, and mineral compositions of Pleurotus ostreatus fruit bodies. The experiment was conducted in a completely randomized design. Data were analyzed using Statistical Package for Social Scientists (SPSS) version 22.0. Means were separated using Turkey’s honestly significant difference at 5% level of significance. Results of the investigation showed that all substrates (CG, DG, and 50% CG + 50% DG) started producing fruit bodies after 15 days. About 50% CG + 50% DG gave the highest number of fruit bodies 297and CG gave the lowest number (184) of fruit bodies. Results on the morphological characteristics of P. ostreatus fruit bodies showed that CG and DG substrates performed better than 50% mixture of CG and DG. The result on the proximate (%) composition of P. ostreatus fruit-bodies showed that P. ostreatus cultivated on all the substrates gave appreciable amount of dry matter (DM), crude protein, and carbohydrate. The ash and crude protein recorded a significant difference (P ≥ 0.05) between the fruit bodies harvested from CG substrate and fruit bodies harvested from DG substrate and 50% mixture of CG and DG substrate. Fruit bodies harvested from DG substrate contained highest quantities of DM with value (91.17±0.09%) and carbohydrates with value (62.74±0.25%). The DM and carbohydrate contents for DG substrate were significantly different (P ≥ 0.05) from values obtained in CG substrate. Result of minerals content of P. ostreatus fruit bodies in all substrates showed that the fruit bodies contained good quantity of minerals such as calcium, potassium, sodium, and phosphorus but copper was in a low quantity. Therefore, farmers who specialize in mushroom cultivation can substitute compliment these common substrates such as rice straw and wheat straw with CG and DG substrates because they are good sources of P. ostreatus fruit-bodies, as well as supporting large number of qualities, nutrient, and fruit-bodies of the mushroom.
Keywords: Chloris gayana, dactylis glomeraata, fruit-bodies, nutritional composition, yield