REFERENCES
1. Lozet J, Mathieu C. Dictionary of Soil Science. 2nd ed. Rotterdam:August AiméBalkema;1991.
2. Bambara LT, Kabore K, Cisse O, Derra M, Zougmore F, Bentil N. Assessment of pollution in agricultural soil and interrelationship between the heavy metals at Paspanga, Burkina Faso. J Environ Sci Toxicol Food Technol 2015;9:84-90.
3. World Health Organization. Environment and Health Risks:A Review of the Influence and Effects of Social Inequalities. Europe:World Health Organization;2010. https://www.euro.who.int/data/assets/pdf_file/0003/78069/E93670.pdf?ua=1. [Last accessed on 2019 Nov 12].
4. Khana MU, Malika RN, Muhammad S, Ullah F, Qadird A. Health risk assessment of consumption of heavy metals in market food crops from Sialkot and Gujranwala Districts, Pakistan. Hum Ecol Risk Assess 2015;21:327-37.
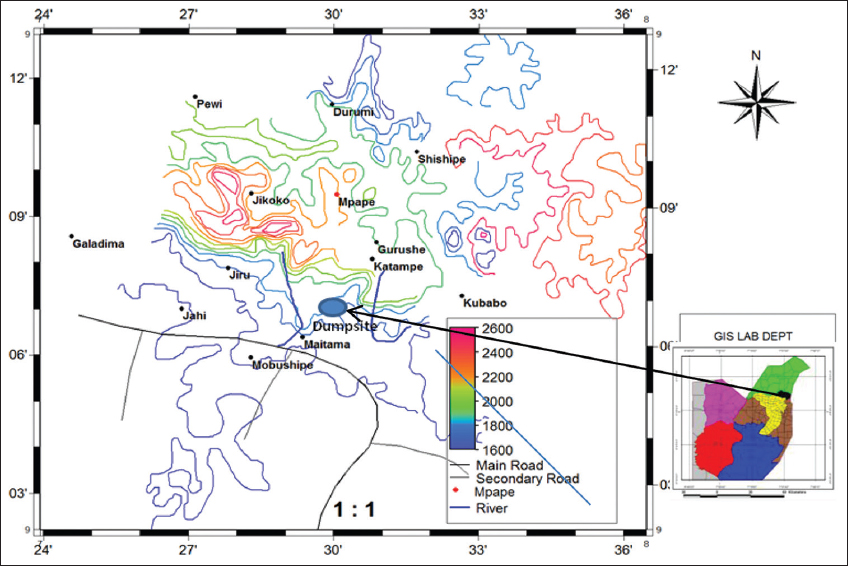
5. Magaji JY. Effects of waste dump on the quality of plants cultivated around Mpape dumpsite FCT Abuja, Nigeria. Ethiop J Environ Stud Manag 2012;5:567-73.
6. Sorme L, Lagerkvist R. Sources of heavy metals in urban wastewater in Stockholm. Sci Total Environ 2002;298:131-45.
7. Magaji JY, Jenkwe, ED. Assessment of the Effects of Spent Engine Oil on Soil Properties and its implication on Plants Growth in Gwagwalada, Abuja, Nigeria. Nigerian Geographical Journal 2017;11:50-66.
8. Elaigwu SE, Ajibola VO, Folaranmi FM. Studies on the impact of municipal waste dumps on surrounding soil and air quality of two cities in Northern Nigeria. J Appl Sci 2007;7:421-5.
9. Mnolawa KB, Likuku AS, Gaboutloeloe GK. Assessment of heavy metals pollution in soils along major roadside areas of Botswana. Afr J Environ Sci Technol 2011;5:186-96.
10. Baba AA, Adekola FA, Lawal A. Trace metals concentration in roadside dust of Ilorin town. Centrepoint (Science Edition) 2009;16:57-64.
11. Salmamanzadeh M, Saeedi M, Nabibidhendi G. Heavy metals pollution in street dusts of Tehran and their ecological risk. J Environ Stud 2012;38:4-6.
12. Wei B, Yang L. A review of heavy metal contamination in urban soils, urban road dust and agricultural soils from China. Michrochem J 2010;94:99-107.
13. Tchounwou PB. Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Mol Clin Environ Toxicol Exp Suppl 2012;101:133-64.
14. Odai SN, Mensah E, Sipitey D, Ryo S, Awuah E. Heavy metals uptake by vegetables cultivated on urban waste dumpsites:A case study of Kumasi, Ghana. Res J Environ Toxicol 2008;2:92-9.
15. Liao X, Chen T, Xie H, Liu Y. Soil as contamination and its risk assessment in the areas near the industrial districts of Chenzhou city, Southern China. Environ Int 2005;31:791-8.
16. Muhammad S, Iqbal J, Akhter G, Shah MH. Fractionation, bioavailability, contamination and environmental risk of heavy metals in the sediments from a freshwater reservoir, Pakistan. J Geochem Explor 2006;184:199-208.
17. Mahmood A, Malik RN. Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab J Chem 2014;7:91-9.
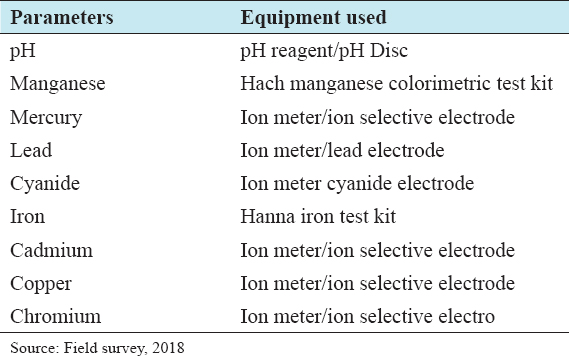
18. Ademoriti CM. Standard Methods for Water and Effluents Analysis. Ibadan:Foludex Press Ltd.;1996.
19. American Public Health Association. System for Analysis of Waste Water. Loveland, Colorado:Hach Company;2000.
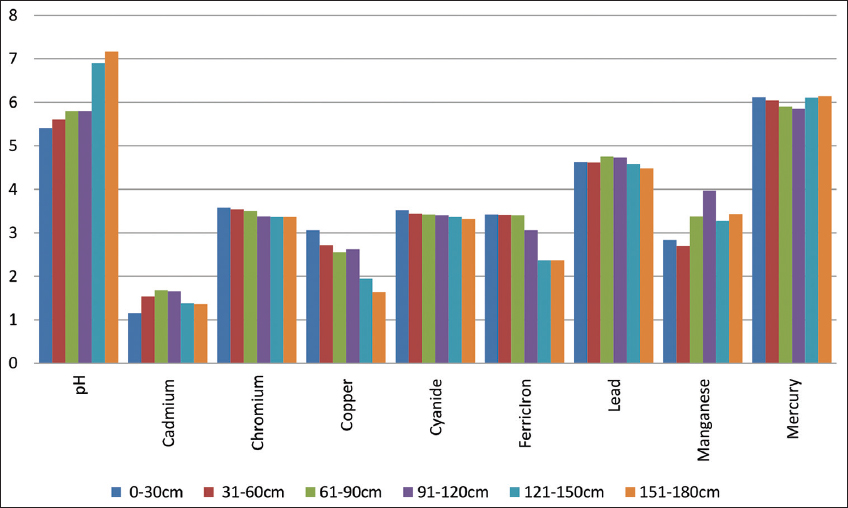
20. Magaji JY. Evaluation of Mpape landfill standard in FCT Abuja, Nigeria. Int J Sci Res Publications 2020;10:589-96.
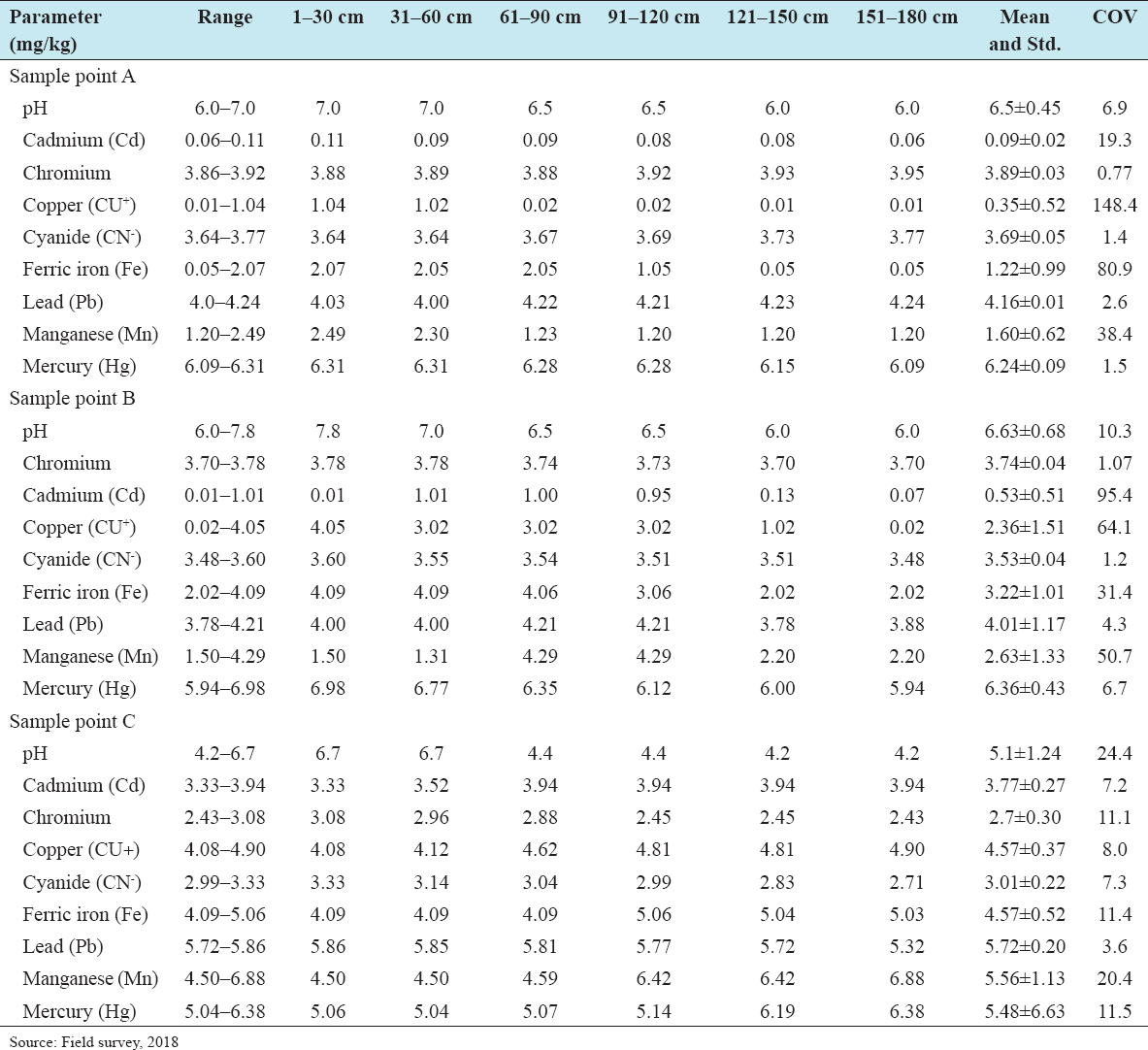
21. Inmaculada R, Angel DT, Forja JM, Abelardo GP. The influence of pH and Salinity on the toxicity of heavy metals in sediment to the estuarine clam Ruditapes philippinarum. Environ Toxicol Chem 2004;23:1100-7.
22. Gabler HE. Mobility of heavy metals as a function of pH of samples from an overbank sediment profile contaminated by mining activities. J Geochem Explor 1997;58:185-94.
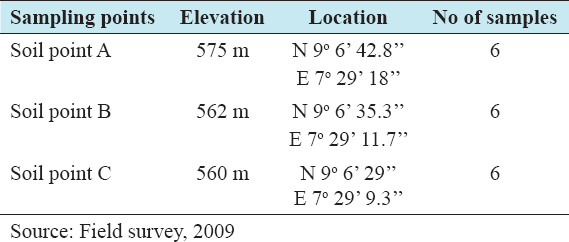
23. Musa SD. An Evaluation of Soil and Crop Conditions on a Waste Dump Site in Anyigba Kogi State. A Ph.D Progress Report to the Department of Geography and Environmental Management. Nigeria:University of Abuja;2009.
24. Mkhabela M, Warman PR. The influence of municipal solid waste compost on yield, soil phosphorus availability and uptake by two vegetable crops, grown in a Pugwash sandy loam soil in Nova Scotia. Agric Ecosyst Environ 2005;106:57-67.
25. Hernando S, Lobo MC, Polo A. Effect of the application of municipal refuse compost on the physical and chemical properties of soil. Sci Total Environ 1989;81-82:589-96.
26. Maynard A. Evaluating the suitability of MSW compost as a soil amendment in field-grown tomatoes. J Compost Sci Util 1995;1:34-6.
27. Zheljazkov JV, Phil W. Source-separated municipal solid waste compost application to Swiss chard and basil. J Environ Qual 2004;33:542-52.
28. Yang ZF, Chen Y, Qian X, Guo L, Zhu HY. A study of the effect of soil pH on chemical species of cadmium by simulated experiments. Earth Sci Front 2005;12:252-60.