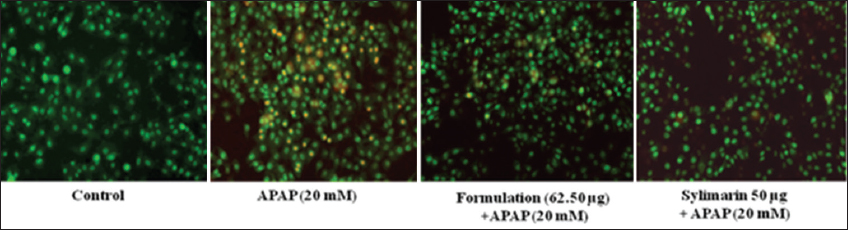
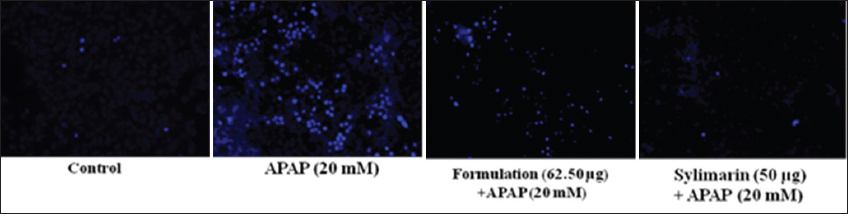
1. Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 2012;56:952-64.
2. Sharma A, Chakraborti KK, Handa SS. Anti-hepatotoxic activity of some Indian herbal formulations as compared to silymarin. Fitoterapia 1991;62:229-35.
3. Subramonium A, Pushpangadan P. Development of phytomedicines for liver diseases. Indian J Pharmacol 1999;31:166-75.
4. Slitt AM, Dominick PK, Roberts JC, Cohen SD. Effect of ribose cysteine pretreatment on hepatic and renal acetaminophen metabolite formation and glutathione depletion. Basic Clin Pharmacol Toxicol 2005;96:487-94.
5. Tan SC, New LS, Chan EC. Prevention of acetaminophen (APAP)-induced hepatotoxicity by leflunomide via inhibition of APAP biotransformation to N-acetyl-p-benzoquinone imine. Toxicol Lett 2008;180:174-81.
6. Song E, Fu J, Xia X, Su C, Song Y. Bazhen decoction protects against acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. PLoS One 2014;9:e107405.
7. Noh JR, Kim YH, Hwang JH, Choi DH, Kim KS, Oh WK, et al. Sulforaphane protects against acetaminophen-induced hepatotoxicity. Food Chem Toxicol 2015;80:193-200.
8. Kumar A. A review on hepatoprotective herbal drugs. Int J Res Pharm Chem 2012;2:92-102.
9. Seeff LB, Lindsay KL, Bacon BR, Kresina TF, Hoofnagle JH. Complementary and alternative medicine in chronic liver disease. Hepatology 2001;34:595-603.
10. Mosmann T. Rapid colorimetric assay for cellular growth and survival:Application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63.
11. Takanashi T, Ogura Y, Taguchi H, Hashizoe M, Honda Y. Fluorophotometric quantitation of oxidative stress in the retina in vivo. Investig Ophthalmol Visual Sci 1997;38:2721-8.
12. Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol 2012;188:3648-57.
13. Satoh T, Enokido Y, Aoshima H, Uchiyama Y, Hatanaka H. Changes in mitochondrial membrane potential during oxidative stress-induced apoptosis in PC12 cells. J Neurosci Res 1997;50:413-20.
14. Jimenez PC, Wilke DV, Takeara R, Lotufo TM, Pessoa C, de Moraes MO, et al. Cytotoxic activity of a dichloromethane extract and fractions obtained from Eudistoma vannamei (Tunicata:Ascidiacea). Comp Biochem Physiol A Mol Integr Physiol 2008;151:391-8.
15. Sabir S, Rocha J. Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro anti-oxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem 2008;111:845-51.
16. Sharma SK, Arogya SM, Bhaskarmurthy DH, Agarwal A, Velusami CC. Hepatoprotective activity of the Phyllanthus species on tert-butyl hydroperoxide (t-BH)-induced cytotoxicity in HepG2 cells. Pharmacogn Mag 2011;7:229-33.
17. Valan M, Britto A, Venkataraman R. Phytoconstituents with hepatoprotective activity. Int J Chem Sci 2010;8:1421-32.
18. Rehman K, Iqbal MJ, Zahra N, Akash MS. Liver stem cells:From preface to advancements. Curr Stem Cell Res Ther 2014;9:10-21.
19. Yang H, Jiang T, Li P, Mao Q. The protection of glycyrrhetinic acid (GA) towards acetaminophen (APAP)-induced toxicity partially through fatty acids metabolic pathway. Afr Health Sci 2015;15:1023-7.
20. Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact 2009;181:15-9.
21. Li CJ, Ma J, Sun H, Zhang D, Zhang DM. Guajavadimer A, a dimeric caryophyllene-derived meroterpenoid with a new carbon skeleton from the leaves of Psidium guajava. Org Lett 2016;18:168-71.
22. Khan H, Ullah H, Nabavi SM. Mechanistic insights of hepatoprotective effects of curcumin:Therapeutic updates and future prospects. Food Chem Toxicol 2019;124:182-91.
23. Subramanya SB, Venkataraman B, Meeran MF, Goyal SN, Patil CR, Ojha S. Therapeutic potential of plants and plant derived phytochemicals against acetaminophen-induced liver injury. Int J Mol Sci 2018;19:E3776.
24. Manov I, Hirsh M, Iancu TC. N-acetylcysteine does not protect HepG2 cells against acetaminophen-induced apoptosis. Basic Clin Pharmacol Toxicol 2004;94:213-25.
25. Raza H, John A. Differential cytotoxicity of acetaminophen in mouse macrophage J774.2 and human hepatoma HepG2 cells:Protection by diallyl sulfide. PLoS One 2015;10:e0145965.
26. Du K, Ramachandran A, Jaeschke H. Oxidative stress during acetaminophen hepatotoxicity:Sources, pathophysiological role and therapeutic potential. Redox Biol 2016;10:148-56.
27. Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. Withaferin-A reduces acetaminophen-induced liver injury in mice. Biochem Pharmacol 2015;97:122-32.
28. Ding Y, Li Q, Xu Y, Chen Y, Deng Y, Zhi F, et al. Attenuating oxidative stress by paeonol protected against acetaminophen-induced hepatotoxicity in mice. PLoS One 2016;11:e0154375.
29. Donatus IA, Sardjoko, Vermeulen NP. Cytotoxic and cytoprotective activities of curcumin. Effects on paracetamol-induced cytotoxicity, lipid peroxidation and glutathione depletion in rat hepatocytes. Biochem Pharmacol 1990;39:1869-75.
30. Lemasters JJ, Qian T, Elmore SP, Trost LC, Nishimura Y, Herman B, et al. Confocal microscopy of the mitochondrial permeability transition in necrotic cell killing, apoptosis and autophagy. Biofactors 1998;8:283-5.
31. Gores GJ, Miyoshi H, Botla R, Aguilar HI, Bronk SF. Induction of the mitochondrial permeability transition as a mechanism of liver injury during cholestasis:A potential role for mitochondrial proteases. Biochim Biophys Acta 1998;1366:167-75.
32. Burcham PC, Harman AW. Acetaminophen toxicity results in site-specific mitochondrial damage in isolated mouse hepatocytes. J Biol Chem 1991;266:5049-54.
33. Bae MA, Pie JE, Song BJ. Acetaminophen induces apoptosis of C6 glioma cells by activating the c-Jun NH(2)-terminal protein kinase-related cell death pathway. Mol Pharmacol 2001;60:847-56.
34. Boulares AH, Zoltoski AJ, Stoica BA, Cuvillier O, Smulson ME. Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol 2002;90:38-50.
35. Wiger R, Finstad HS, Hongslo JK, Haug K, Holme JA. Paracetamol inhibits cell cycling and induces apoptosis in HL-60 cells. Pharmacol Toxicol 1997;81:285-93.