INTRODUCTION
Activated carbon is an important industrial adsorbent used extensively in precious metal recovery. The major raw materials for its production include coal, coke, peat, and agricultural waste such as coconut shells and palm kernel shell (PKS) which are readily available in Ghana in commercial quantities.[1-4]
There are two main modes of producing activated carbon, namely the physical or steam activation and the chemical activation. In the latter, a precursor usually of plant origin is impregnated with a chemical activation agent such as HNO3 and ZnCl2 and then followed by carbonization and activation which takes place simultaneously.[5]
Ghana, formerly the gold coast, is well known for its mineral resources, especially gold. At the core of modern gold mining operations is the role of activated carbon. The production of activated carbon for a specific usage or targeted industry, the gold mine industry is, therefore, an economically viable venture worth pursuing in Ghana.
In this study, granular activated carbon is prepared from PKS using crude potash leached from cocoa husk ash as activating agent to derive economic benefit – gold adsorption operations and also help in resolving the environmental issues with regard to agro-waste disposal. The product was then characterized and the independent t-test was carried out to ascertain whether a significant difference exists between the fresh carbon activity and attrition percent of samples impregnated at room temperature and 85°C.
MATERIALS AND METHODS
Raw Materials and Reagents
PKSs were obtained from a PKS dumpsite – stadium road, Obuasi in the Obuasi Municipality of Ashanti Region, Ghana. Cocoa pod husk was obtained from Akrokerri in the Adansi North District of Ashanti Region, Ghana. The husks were washed thoroughly to remove dirt, dried, combusted, and leached with distilled water to obtain the activating agent, crude potash.
Raw Material Analysis
The suitability of the precursor for activated carbon production was ascertained by carrying out proximate analysis. The American Society for Testing and Materials (ASTM) D3173, ASTM D3174, and ASTM D3175 were used to determine the moisture content, ash content, and volatile content, respectively.[6]
Typically, the mass loss of 1 g of pulverized PKS expressed as a percentage when the biomass in subjected to heating at 110°C for an hour gives the moisture. The ash content is obtained by heating 1 g of dried pulverized palm kernel at 500°C for an hour and increased to 700°C and maintained at that temperature for 2 h. The loss in mass expresses as a percentage gives the ash content. The volatile matter component is obtained by expressing the mass loss as a percentage when 1 g of pulverized PKS is heated at 930° for 7 min.
Double indicator titration method was employed to determine the crude potash content of the leachate.[7]
The raw materials analysis was carried out at the chemical engineering department laboratory at Kwame Nkrumah University of Science and Technology, Kumasi, Ghana.
Preparation of Activated Carbon
The PKS was washed severally to remove all foreign particles, dried on a clean platform for 3 weeks, crushed, and sieved to particle sizes between 1.2 mm and 2.4 mm. The leached potash was used to impregnate the precursor at IRs of 0.1, 0.5, and 1.0 (1.5 and 2.0 inclusive in the case of activation at 800°C). The IR was obtained using the relation:
I.R. = (Mass of Crude K2CO3 in Leachate)/(Mass of PKS)[8,9]
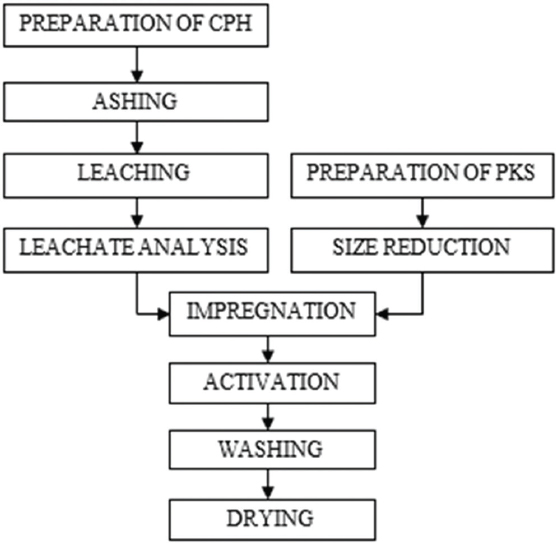
The samples were impregnated at room temperature and 85°C for 12 h, dried at 110°C, and then carbonized/activated in a muffle furnace heated at a rate of 8°C/min until the final temperature and maintained at that temperature (600°C–800°C) for 2 h, allowed to cool, washed sequentially with distilled water to remove residual chemicals, and then dried at 110°C before characterizing. A summary of the stages involved in the production of activated carbon is shown in Figure 1.
Figure 1: Block diagram for activated carbon production
Characterization of Carbon
The produced activated carbon was characterized as per two major parameters of carbon used in gold mine operations – fresh carbon activity and attrition (hardness).
Fresh carbon activity
About 9 g of fresh carbon was added to a solution of 0.1% sodium cyanide and about 10 ppm of the gold standard solution in a Winchester bottle, whirled for about 60 min on a mechanic roller and the solution analyzed with an atomic absorption spectrophotometer machine to determine the Au left in the solution. Fresh carbon activity test was carried out at Intertek Mineral Laboratory, Tarkwa, a commercial mineral laboratory in Ghana.
Percent attrition (hardness)
The attrition level was determined using a wet attrition method. Marshall and Johns, 1996, and Toles et al., 2000, methods were adapted.[10,11] About 1 g of activated carbon was put in 0.1 M acetate buffer and stirred at 500 rpm for 2 h with a 1.5-inch stir bar and screened on 0.30 mm sieve. The retained samples on the sieve are dried and the loss in mass computed as the percent attrition.
Statistical analysis of results
The independent t-test was used to analyze the fresh carbon activity and the percent attrition of the samples produced in the same manner (subjected to the same IR and activation temperature) but different treatment (impregnation temperature). The analysis was carried out at 95% confidence level. P value (sig. two-tailed value) higher than α-value (0.05) means no significant difference exists between the quality parameter of the samples impregnated at room temperature and those impregnated at 85°C and vice versa.
RESULTS AND DISCUSSION
Precursor Appraisal
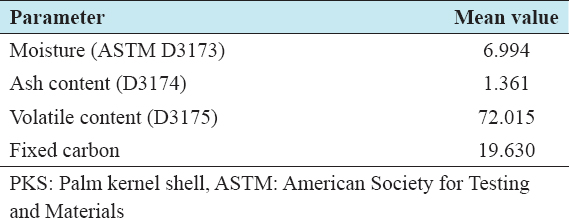
The suitability or otherwise of the precursor for the production of activated carbon was ascertained by carrying out a proximate analysis The results of the proximate analysis on PKS are summarized in Table 1. The results reveal a low ash content of about 1.361% and a relatively high fixed-carbon content of about 19.630% similar to those observed for other biomass materials used for activated carbon production.[12-14] Hence, PKS is a good raw material for activated carbon production.
Table 1: Summary of proximate analysis on PKS
Produced Activated Carbon Analysis
Fresh carbon activity
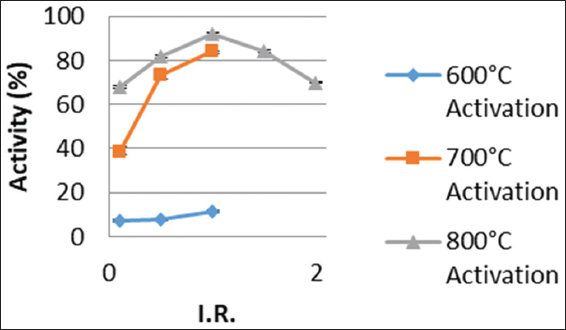
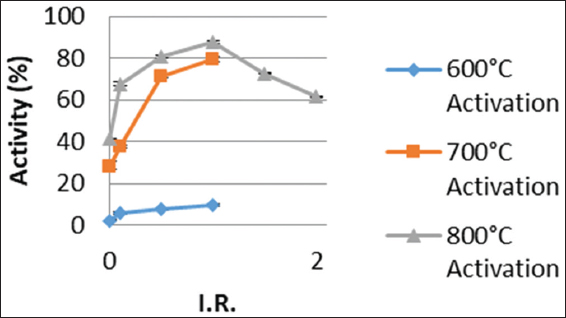
The fresh carbon activity, an important quality parameter of activated carbon in gold adsorption operations, for both samples impregnated at room temperature and those impregnated at 85°C increased with increasing activation temperature from 600°C to 800°C as shown in Figures 2 and 3. This observation, of relatively higher activity values occurring for activations at 700°C and 800°C, may be due to increased devolatilization occurring at such relatively high temperatures resulting in the creation of more pores, leading to an increase in surface area and for that matter fresh carbon activity.[15,16] The leachates from the cocoa husk ash, crude potash used as the chemical activation agent also played a crucial role in the recorded fresh carbon activity. For low IRs such as 0.1, the degree of activation of pores in the internal structure of the carbon is low and increases as the IR increases. However, for IRs above 1.0 as observed for activation at 800°C, the activity decreased as this could probably be due to excessive activation which leads to a collapse of the internal pore structures.[17,18]
Figure 2: Effect of impregnation ratio and activation temperature on fresh carbon activity at 85°C impregnation temperature
Figure 3: Effect of impregnation ratio and activation temperature on fresh carbon activity at room temperature impregnation
Percent attrition (hardness)
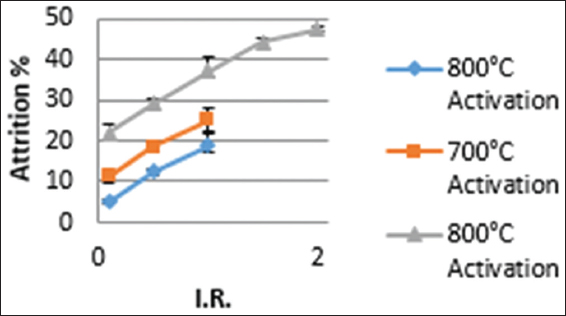
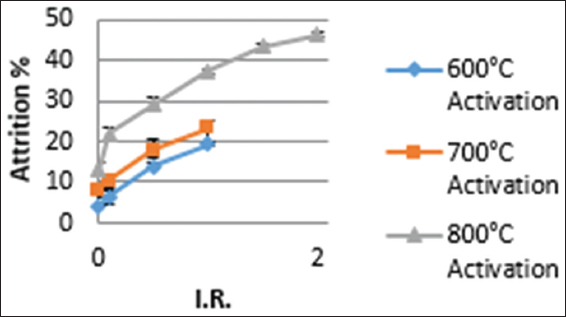
The percent attrition of activated carbon is a quality parameter that determines the hardness of the carbon. Activated carbon with low percent attrition is desirable for rigorous mechanical usage such as gold adsorption operations. The observed percent attrition for the prepared activated carbon generally increased with increasing activation temperature for both samples impregnated at room temperature and those impregnated at 85°C [Figures 4 and 5]. Similar trend was observed by Ravichandran et al., 2018.[12] This observation reiterates the fact that biomass generally disintegrates with increasing temperature.[19] The analysis of the effect of impregnation temperature on attrition percent is shown in Table 2. Higher activation temperatures lead to a collapse of internal pore structures, leading to a generally structurally weak carbon that cannot withstand rigorous activities.[2]
Figure 4: Effect of impregnation ratio and activation temperature attrition percentage for impregnation at 85°C
Figure 5: Effect of impregnation ratio and activation temperature on attrition percentage at room temperature impregnation
Table 2: Difference in attrition of samples impregnated at 85°C and room temperature
Statistical Interpretations
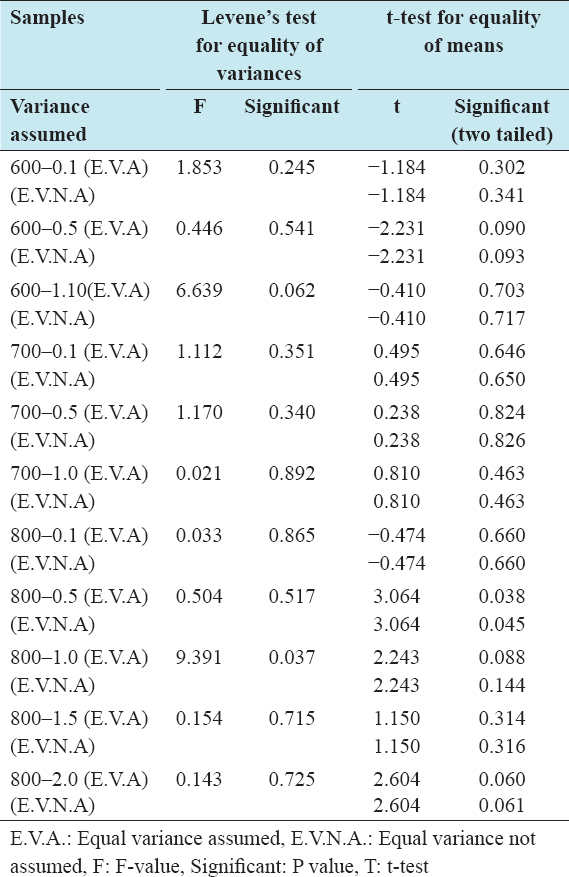
The independent t-test analysis on the fresh carbon shows that for samples activated at 800°C with IR higher than 1.0, there was a significant effect of impregnation temperature on fresh carbon activity as the recorded P values (0.009, 0.000, and 0.000) were smaller than 0.05. For all other samples, the converse is true. In all cases, equal variance was assumed. Details of the statistical analysis on the effect of impregnation temperature on fresh carbon activity is shown in Table 3.
Table 3: Difference in activity of samples impregnated at 85°C and room temperature
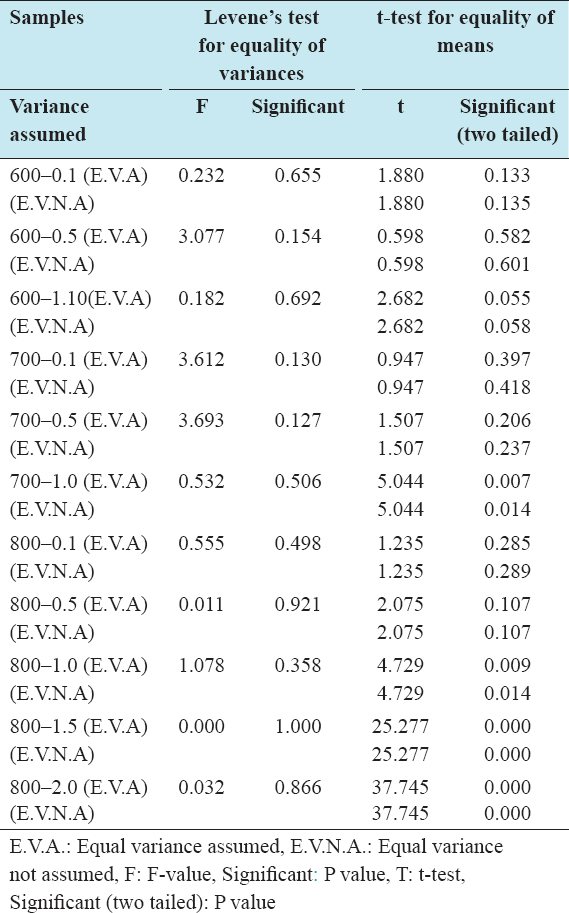
The independent t-test analysis for the percent attrition reveals that with the exception of samples activated at 800°C and with an IR of 1.0, equal variance was assumed for all sample analysis. There proved to be no effect of impregnation temperature on percent attrition except for samples with IR of 0.5 and activated at 800°C.
CONCLUSION
PKSs, a major agro-waste in Ghana, can be used effectively as raw material for the production of activated carbon using leachates from cocoa husk ash (cruse potash) as substantial effect on the hardness of the produced activated chemical activation agent. The temperature for chemical impregnation of precursor has no carbon. Impregnation temperature has effect statistically on the fresh carbon activity with IR of 1.0 being the optimum chemical IR for the best fresh carbon activity.