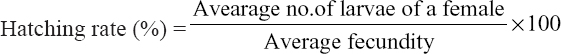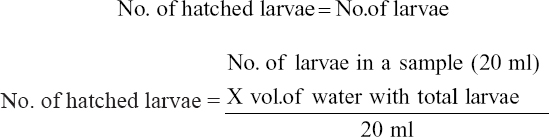INTRODUCTION
The freshwater prawn, Macrobrachium rosenbergii (de Man, 1879), locally known as “Galda,” is native in some tropical and subtropical countries of the world[1] including Bangladesh. The giant freshwater prawn M. rosenbergii has a wide distribution throughout the Indo-Pacific region and most favored for farming in tropical and subtropical areas of the world.[2,3] It is also available both in freshwater and brackish water environment in Bangladesh. Global production of this prawn has increased from 130,689 tons in 2000 to 203,211 tons in 2011.[4] The freshwater prawn, M. rosenbergii farming plays an important role in the economy of Bangladesh. Bangladesh is blessed with giant river prawn (M. rosenbergii) farming due to its favorable resources, including vast inland freshwater and adjacent brackish water areas, geographic location, and agroclimatic conditions. The freshwater prawn, M. rosenbergii is an indigenous species in Bangladesh. Twenty-four species of freshwater prawns including ten species of Macrobrachium are found in Bangladesh,[5,6] of which only M. rosenbergii is commercially cultured. Bangladesh was the third largest global producer of giant freshwater prawn in 2010.[7] It is cultured mainly in Bagerhat, Khulna, Jessore, Satkhira, Pirojpur, and Gopalganj district. Now its farming has been expanded in new areas such as Patuakhali, Barishal, Barguna, Madaripur, Faridpur, Noakhali, and Mymensingh.
Now, it is cultured in a total of 77 countries[8] especially in Asia (China, Thailand, Vietnam, Taiwan, Bangladesh, and India) and in Latin America (Ecuador and Brazil). During the last three decades, its farming has attracted considerable attention for its market price and export potential.[6] Total export of 29,713 mt frozen shrimp and prawn in 2000–2001 fiscal years has increased to 51,599 mt in 2009–2010.[9] The contribution of freshwater prawn as a single species is approximately 25–30% of the total exported frozen shrimp and prawn of the country. Thus, within the overall agro-based economy in Bangladesh, freshwater prawn (M. rosenbergii) farming is currently one of the most important sectors of the national economy.[6] There are existing scopes for prawn production in numerous freshwater bodies throughout Bangladesh. Due to lack of stockable sized seeds, prawn farmers are still facing difficulties in culturing prawns. Due to a steady demand of prawn seeds all over the world, countries such as Thailand, Indonesia, Malaysia, Taiwan, Honduras, Sri Lanka, Vietnam, China, and Japan are trying to produce the needful prawn seeds for the past four decades.
The peculiarity of prawn culture is that prawn is grow, mature, egg fertilize and even hatch in the freshwater environment, but after hatching, larvae cannot survive or grow up to post-larvae stage without brackish water. Hence, it is well known that salt water (brine) is the key materials for establishing Golda hatchery to produce postlarvae. Therefore, prawn farming is being established in and around coastal areas. Now, farmers of the northern part of the country are very much interested to the culture of prawn and in addition intensification of prawn culture in an existing farm in coastal areas, and further expansion in new farms would increase the demand for postlarvae. Besides, the establishment of prawn hatcheries in inland areas it is required to ensure countrywide supply of prawn seed. However, when establishing of prawn hatchery in the northern part of the country brine collection from the coastal areas or southern part is the main prerequisite. However, availability and collection of brine from the southern part to the northern part are time consuming and costly for the prawn hatchery owners in due time. Hence, the hatchery owner’s desire a technology like replacement of brine by crude salt, which may have to reduce the operating cost for the Pl production in the hatchery. To overcome the problem, it is urgently needed to establish a cost-effective Bangladesh Fisheries Research Institute developed backyard prawn hatchery in the freshwater area especially Northern part of Bangladesh.
Prawn farming (in gher) in the Southwest region of Bangladesh is taken as a traditional custom and alternative income source. Despite immense potential, expansion of prawn farming in the country is limited due to several constraints. The most important is insufficient and unreliable supply of prawn seed due to the nonexistence of prawn hatchery in both coastal and inland areas, initial mass mortality of larvae in hatchery and also mortality of PL in grow-out ponds, lack of prawn grow-out management techniques. Aquaculture production of prawn depends on the quality of PL stocking from nature and hatcheries. However, our farmers do not get sufficient and quality PL from hatcheries due to lack of proper maintenance of the hatcheries such as proper water quality and environment management. Before stocking in the grow-out pond, nursery techniques of newly produced PL for 1–3 months are an important step in freshwater prawn aquaculture. On the other hand, due to lack of proper nursery management of PL in pond, farmers are not getting higher survival and production. Grow-out farmers prefer to stock their ponds with juveniles rather than PL. Under the above circumstances, the project was proposed to find out the alteration technique of brine by crude salt from salt pan wholly or partially in the backyard Golda hatchery for the production of Golda seed.
METHODOLOGIES
Prawn larvae rearing technique were conducted in the Backyard hatchery with various saline water under three different treatments, namely brine (T1), crude salt (T2), and mixture of brine and crude salt with the ratio of 1:1 (T3) and each having three replications. The materials methods were as followed:
Collection of Brine and Crude Salt
Brine and crude salt were collected from Pekua Upazila of Cox’s Bazar. Collected brine was put in a cemented tank to settle dawn the sediments presence in the brine water. Then, the settled brine needs to be treated (described next section) for cleaning and free from pollution before use in hatchery. Crude salt was also prepared up to required salinities diluted in freshwater [Figure 1].
Figure 1: (a-d) Collection of brine and crude salt from Pekua Upazila of Cox’s Bazar
Collection of Broods
Fifty wild berried females of freshwater prawn, M. rosenbergii (average wt. 80–100 g) were collected from the Bolleshari river of Pirojpur. Selection of berried females [Figure 2a] should be done carefully on the basis of some characters such as broods are healthy and active; well pigmented, with [Figure 2b] no missing appendages or other injury, and carrying large egg masses. The ripeness of the eggs [Figure 2c] is also essential. As the eggs ripen, their color changes from dark orange to brown and finally to grayish-brown a few days before hatching. Berrieds those carrying brown to grey eggs were the best ones to bring in the hatchery as their eggs hatched out within 2–3 days. Collected broods were acclimatized and kept in cisterns filled up with fresh-water in the hatchery, and continuous aeration was provided, and the fish were fed with commercially available mega feed (35% protein) at the rate of 2–4% BW twice a day.
Figure 2: (a-c) Collected broods from Bolleshari river of Pirojpur
Hatching and Larvae Rearing Techniques in Brine and Crude Salt
Experimental design
The experiment was conducted in six fiberglass tanks at the hatchery during June 2015. Continuous aeration was provided in each tank through air blower. The tanks (250 L water capacity each) were divided and designated under the three treatments such as brine (T1), crude salt (T2), and mixture of brine and crude salt with the ratio of 1:1 (T3) and each treatment having two replications in case of hatching. The hatching tanks were filled up with various saline water made from treated brine, crude salt water and mixture of both brine and crude salt water of 6 ppt. The larvae rearing tanks were also divided and placed under the three treatments such as brine (T1), crude salt (T2), and mixture of brine and crude salt (1:1) (T3) and each treatment having three replications.
Preparation of saline water from brine and crude salt
The water was treated/disinfected with bleaching powder (calcium hypochlorite) at a dose of 10–12 ppm for the killing/destruction of the harmful organisms/bacteria and aeration was provided vigorously for 1 day to eliminate the smell of chlorine. After 24 h, the water was again treated with sodium thiosulfate at a dose of 10–12 ppm to eliminate the access chlorine and again aerated vigorously for 1 day and to keep stable for 1 day to settle down the suspended particles. The supernatant water was transferred to the sand filter bed for physical filtration. After filtration required amount of freshwater was mixed with brine to make 12 ppt saline water. Then, saline water of 12 ppt was stored in a reserved tank [Figure 3a]. To increase the sodium salt in water quality, the chelating agent ethylenediaminetetraacetic acid added 5–10 ppm which may improve the performances of larvae growth. Then, the treated water was passed through the sand filter and stored in the reserve tank [Figure 3b]. This treated water was transferred into the larvae rearing tanks [Figure 3c]. The saline water made from crude salt was also treated followed the same procedure as brine.
Figure 3: (a-c) Different treatment of brine and crude salt in tank in the hatchery
The necessary materials such as pipes, siphoning pipes, bowl, nets, gravel, cover, and others for hatchery operation were disinfected by soaking in a solution of 200 ppm formalin for 30 min.
Hatching and larvae rearing in saline water made from brine and crude salt
The 18 nos. berried females [Figure 4a] were collected from the cisterns and disinfected for 1 h with 25 ppm formalin. Then, the disinfected females were kept in 6 hatching tanks [Figure 4b] which having 6 ppt saline water made from brine, crude salt, and mixture of brine and crude salt. The females were fed with mega feed at the rate of 5–10% of their body weight. The females were [Figure 4c] hatched after 2 days in every hatching tank. Hatching of larvae started in the afternoon and it continued up to the midnight. After hatching females were transferred from the hatching tank. The larvae were collected by scoop net in the morning and were kept in separate plastic bowls [Figure 4d] with sufficient aeration [Figure 4e]. Then, the numbers of larvae were counted by volumetric method following standard procedure. A larvae sample of 20 ml from the bowl was taken with a small beaker, the water was gradually dropped out, and the number of larvae was counted. This process repeated for 3 times for the counting of the average number of larvae in 20 ml water. Finally, the treatment-wise total number of hatched larvae in each bowl was counted according. The number of produced larvae of individual berried females and hatching percentage was calculated by the following formula:
Figure 4: (a-e) Brood female, hatching tank, and larvae rearing in fiberglass tank
No. of hatched larvae = No. of larvae
The larvae were reared under three different saline water treatments, namely T1 (brine), T2 (crude salt), and T3 (brine:crude salt, 1:1). The newly hatched larvae were collected and disinfected at 200–250 ppm formalin bath for 30 min and then stocked under three respective treatments in a total of nine rearing tanks each containing 200 L of 12 ppt saline water and at a density of larvae 100/L.
The larvae were fed with brine shrimp Artemia nauplii [Figure 5a] thrice a day at 3–5 nos/ml at 08.00 h, 16.00, and 24.00 h for the first 10 days. After 10 days, the larvae were fed with a prepared feed called egg custard [Figure 5c] made of a mixture of 30% egg yolk, 25% milk powder, 35% clam/fish muscle, 6% arrowroot, 3.5% yeast powder, 0.2% oxytetracycline, and 10 drops of multi-vitamin at the rate of 200% body weight, 3–4 times a day and Artemia nauplii [Figure 5b] 2–5 nos/ml in the night. The uneaten feed, molted shell, excreta, and other wastes were siphoned out before every feeding time. Water quality parameters of larvae rearing tanks [Figure 5d] such as temperature, salinity, dissolved oxygen, pH, alkalinity, and un-ionic ammonia were recorded every day. The water quality parameters kept within the range of tolerance such as temperature at 28–30°C, oxygen at 4.7–7 mg/l, pH at 7.5–8.5 ppm, and salinity at 12 ppt, and ammonia free. About 100% of water was exchanged at 3 days’ interval. Larval developmental stages were observed under a microscope. Water quality parameters were monitored every alternate day and mortality of the larvae was counted regularly and results were presented.
Figure 5: (a-d) Artemia and custard feed for larvae
RESULTS
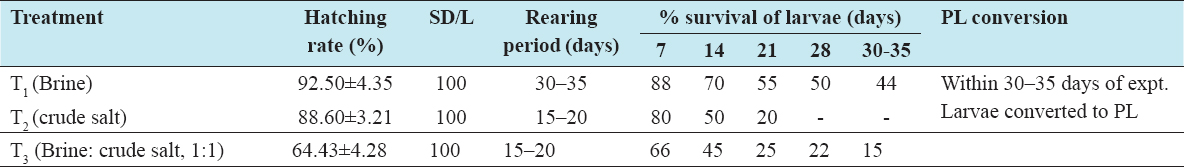
The experiments were conducted as per the design of the project in the materials methods section. Details results of the hatching rate, survival and PL conversion under three different treatments such as brine (T1), crude salt (T2), and mixture of brine and crude salt with the ratio of 1:1 (T3) are shown in Table 1. In the hatching tank, all berried females started spawning after 48 h and continued up to further 48 h. Hatching rates were recorded 92%, 88%, and 64% under treatments T1 (brine), T2 (crude salt), and T3 (brine: crude salt), respectively.
Table 1: Details of hatching, stocking, and survival rate of larvae under different treatments
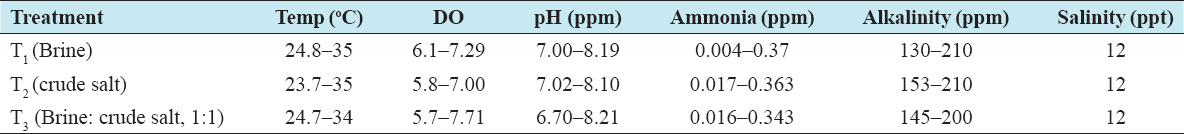
The results of the average survival rates of larvae up to 30–35 days are presented in Table 1. The survival rate of larvae was observed to vary from 15% to 46% under different treatments. The highest rate of survival (44%) was recorded in treatment T1, and the lowest (15%) was in T3, and no larvae survived in T2. The cause of such variation occurred may cause of sudden decreased in temperature and the un-ionic ammonia present in water. Mass mortality was appeared in T2 and T3 while the larvae attain to metamorphosis to PL stage which might possibly due to sudden fall down of water temperature. It was found that the movement of larvae was very active during the age of 20–30 days. The values of physicochemical parameters such as temperature, salinity, pH, alkalinity, and un-ionic ammonia larvae rearing tank were more or less the same in all the treatments; all the results are shown in Table 2.
Table 2: Ranges of physicochemical parameters of the rearing tank under different treatments
The temperature, DO, pH, ammonia, and alkalinity ranged from 23.7 to 35°C, 5.7 to 7.71, 6.70 to 8.21, 0.004 to 0.37, and 130 to 210, respectively, in all the treatments throughout the research period in larvae rearing tank. However, the temperature of water varied more during the experiment which is the vital factor in larvae rearing and might be temperature is one of the major causes for the less survival rate of larvae. Furthermore, the level of ammonia was more. Besides, these other water quality parameters were within the optimum range for larvae rearing. The saline water made from the mixture of brine and crude salt with freshwater for the use of M. rosenbergii hatcheries should be 12–16 ppt, have a PH of 7.0–8.5 and contain a minimum dissolved oxygen level of 5 ppm. Both fresh-water and seawater used for hatchery purposes should have a pH and a temperature as close as possible to the optimum range. Aeration always provides a source of dissolved oxygen. Hydrogen sulfide and chlorine must be absent, and a higher level of nitrite and nitrate nitrogen must be avoided. The characteristics of water found suitable for use during freshwater prawn hatcheries operation are shown in Table 2 except temperature and ammonia. Saline water was used in larvae rearing tank in re-circulation systems in every treatment. The re-circulate system of water may reduce the problems caused by water pollution, parasites, and the predators in the larval rearing tank.
DISCUSSION
The life cycle of giant freshwater prawn M. rosenbergii is being completed both in fresh and brackish water. The larvae of M. rosenbergii need 12–14 ppt saline water for the development of different larval stages to postlarvae. Larvae developed in brackish-water areas migrate to freshwater, shortly after metamorphosis to the post-larval stage, where they grow and get matured. Hence, it is well known that salt water (brine) is the key factors for establishing Golda hatchery to produce postlarvae. Therefore, prawn farming is being established in and around coastal areas. Establishment of prawn hatcheries in inland areas it is required to ensure supply of seawater for making saline water for larvae rearing. However, when establishing of prawn hatchery in the northern part of the country brine collection from the coastal areas or southern part is the main prerequisite. However, availability and collection of brine from the southern part to northern part are difficulty and more cost in trucking seawater and time consuming for the prawn hatchery owners in due time. These problems may be minimized if the larvae rearing saline water can be instantly made from crude salt. Hence, the purpose of the present research was to find out the saline water reconstituted from crude salt and its combination with diluted saline solution in rearing of M. rosenbergii larvae under captive conditions.
The research was carried out to compare the possibilities of using 100% brine, 100% crude salt, and combination of brine and crude salt (50%:50%), under treatments T1, T2, and T3, respectively, in larvae rearing techniques. The larvae rearing techniques were similar, as followed in the present experiment. Details results of the hatching rate, survival, and PL conversion under three different treatments such as brine (T1), crude salt (T2), and mixture of brine and crude salt with the ratio of 1:1 (T3) are shown in Table 1. In treatment, T1 where the brine was used for larvae rearing, 44% of stocked larvae metamorphosed to postlarvae. No larvae in 100% crude salt metamorphosed to postlarvae and they started to die after the 3rd day of the hatching day, the mortality rate increased gradually and all were died after 21 days. The movement of larvae was slow and they were not more active to taking food and colors of larvae were whitish. In treatment, T3 where the saline water was a combination of brine and crude salt, only 15% of stocked larvae metamorphosed to postlarvae. However, a combination of 50% brine and 50% crude salt showed the result in a quite significant rate of survival (15%) of larvae to postlarvae. Yambot and Cruz[10] stated that the survival rate of M. rosenbergii postlarvae ranging from 2.88% to 11.48% with an average of 6.71% while they used a combination of sea salt, deionized freshwater, and Chlorella culture (green water). Maybe this percentage of survival rate was found due to an advantages use of green water in larvae culture.[11] The survival data of M. rosenbergii larvae obtained under different treatments of brine and crude salt reveal that 50% of brine solution could be replaced with a crude salt solution though survival rate is very low, this might be due to fluctuations of water quality parameters especially fall dawn of water temperature and ammonia. On the other hand, no larvae survived when they reared in only saline water which was made from crude salt. This research showed that either brine alone or 50% replacement of brine with the crude salt solution can be used as suitable for larvae rearing technique. Yambot and Cruz[10] stated that the sea salt alone cannot be used in larvae rearing of M. rosenbergii up to postlarvae. It is assumed that as M. rosenbergii is developing out of the sea,[12] as it requires a salinity of 10%–14% in closing the larval cycle.[13] Therefore, it is probable that the complex nutrient and mineral constituents of the natural seawater regulate the growth and survival of larvae. However, the nutrient and mineral constituents of saline water made from crude salt are not the same as like in seawater. When crude salt dissolved in water, it breaks up into Na and Cl ions with equal amounts of positive and negative ions.[14] Therefore, most of the ions except Cl and Na are lost in the process of salt preparation from seawater.