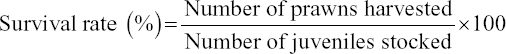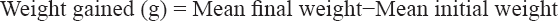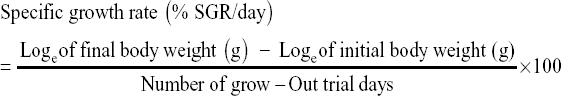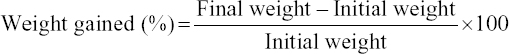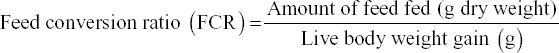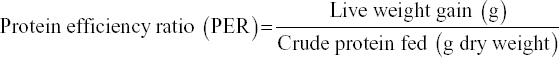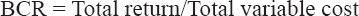INTRODUCTION
Freshwater prawn (Macrobrachium rosenbergii) is one of the aquaculture species attractive to the farmers of Southwestern Bangladesh due to its high price and demand in global markets.[1] Cost-effective, good quality feed is one of the most critical inputs for farming to grow the high-quality prawn demanded by the international market and to optimize the economic return from farming. Feed accounts for 60–80% of the variable costs in intensive systems and 30–60% in semi-intensive systems for feed and fertilizer.[2] Feed cost represents 15%, 25%, and 33%, respectively, for extensive, improved extensive, and semi-intensive prawn farming systems in Bangladesh.[3]
There are more than 100 national and regional private fish feed companies in Bangladesh, producing an estimated 1 million tonnes of aquaculture feed each year.[4] Additional 0.3–0.4 million tonnes of feed are produced by small village level enterprises. The larger feed companies mainly supply medium- to large-scale farmers through well-organized supply chains. However, small-scale farmers in remote areas either cannot afford to buy feed from the larger companies or do not have access to their marketing network. Only around 5–10% of the feed produced by larger national and regional feed companies reaches small-scale farmers in remote areas, supplied through intermediary traders.[4] Most small-scale farmers use feeds such as poultry feed, freshwater apple snails (Pila globosa) collected from the wild, a practice which is not sustainable, or feeds made of a variety of locally available ingredients, such as agriculture by-products. These do not provide a balanced diet and are not suitable for producing quality prawn or satisfactory economic returns.[3,5]
The price of prawn feed produced by the large-scale companies has increased significantly in recent years as prawn farming has become more widespread in Southwest Bangladesh. Although most feed ingredients are locally available and some fish meal is locally produced, much of the demand for fish meal and meat-and-bone meal (MBM) is met by imports, including from China and the EU. Some questions have been raised about the quality and ethics of using imported MBM in prawn feed.[5]
In Bangladesh, the main ingredients used for fish feed production are rice bran, wheat bran, maize, soybean meal, mustard oil cake, fish meal, and MBM. Local and imported fish meal is used as a protein source, manufactured from a variety of fish, crabs, and other aquatic animals, and is variable in nutritional composition.[3] However, in recent years with the support of donor-funded projects in the southern districts of the country, production of various plant protein sources has been promoted, integrated with the Rice Farming System - including hybrid maize (Zea mays) and sunflower (Helianthus annuus). Increased production of maize and sunflower seed has created alternative feed ingredient options for small-scale aquaculture farming. Oil-extracted sunflower cake (SFC) contains 27.8–37.4% crude protein, which may vary with seed quality and processing.[6] SFC can be a good replacement for other plant protein sources such as soybean meal, mustard oil cake, and fish meal due to its competitive nutritional value and pricing.
A previous study during 2014 demonstrated that feed containing 50% SFC inclusion can replace a substantial portion of the fish meal, soybean meal, and mustard oil cake typically used in freshwater prawn diets without compromising economic return and carcass quality.[7] The experiment was conducted with diets formulated by different inclusion rates (30%, 40%, and 50%) of SFC and compared with a control diet formulated by fish meal (25%), soybean meal (16%), and mustard oil cake (16%). The best performing sinking pellet feed with an inclusion of 50% SFC could reduce fish meal, soybean meal, and mustard oil cake content to 13%, 8%, and 10%, respectively, without reducing economic return and carcass quality. However, the performance of that best performing diet was not compared with any commercial feeds. The main objective of the current study was to make that comparison with two widely used commercial feeds (Quality and Titas) were selected as the commercial feeds to be used in the comparative trial. Quality feed is a renowned brand, one of the leading national feed companies, and Titas feed is produced by a regional company active in the southwestern region, especially in Khulna, Satkhira, and Bagerhat districts.
MATERIALS AND METHODS
The experiment was conducted in the same farmer’s gher (pond), where the previous trial to evaluate the performance of feeds formulated with different levels of SFC inclusion in freshwater prawn grow out was conducted.[7] The freshwater pond was in Phultala Upzila of Khulna District in Bangladesh. The experiment was conducted for 100 days, stocking juveniles on June 27, 2015, and harvesting on October 6, 2015.
Pond Preparation and Stocking
The total area of the pond was estimated as 2376 m2, of which 1388 m2 was used for the experiment. To maintain a minimum water depth of 90 cm, approximately 15 cm of soil was removed from the pond bottom. The area required for the study was then separated from the larger pond by installing a bamboo fence coupled with a Fine Mesh Blue Nylon Net along the North–South Axis. The separated section (1388 m2) was then divided into nine equal compartments each of 154 m2, allowing 150 m2 effective water area in each after deduction of the area lost due to the fencing on the four sides. Finally, the entire peripheral dike was fenced with Blue Nylon Net to prevent predation. Each compartment was easily accessible from the dikes so that activities including stocking, feeding, liming, growth sampling, and water quality monitoring could be readily carried out. Each of the three compartments used for the three replications (R1, R2, and R3) for a specific feed (Diet 1 “Q - Quality feed”, Diet 2 “T - Titas feed,” and Diet 3 “W - experimental feed with 50% SFC”) was randomly assigned and marked with a signboard (QR1, QR2, and QR3; TR1, TR2, and TR3; and WR1, WR2, and WR3).
The nine compartments in the pond were prepared following the methodology used in the previous experiment.[7] Rotenone powder (9.1% strength) was applied at the rate of 2.03 g/m2 (20.34 kg/ha) per meter of water depth, followed by liming after 5 days (with CaCO3) at the rate of 25 g/m2 (250 kg/ha). The pond was filled to 90 cm depth by pumping rainwater from an adjoining canal. Before the stocking of juveniles, shelters for molting prawn consisting of two dried palm leaves were vertically laid in each compartment. The molting prawns hide in the shelters for protection until their shells harden, and thus, the shelters play an important role in reducing cannibalism and increasing productivity.[8]
Freshwater prawn juveniles collected from a local prawn nursery (average length of 6.7 cm and weight of 2.47 g) were stocked on June 27, 2015. The nursery operator reported that the post larvae (PL) had been collected from riverine sources and reared for approximately 30 days. The freshwater prawn juveniles were stocked in each compartment at the rate of 1.5 juveniles per square meter of water area. Before stocking, 10% of the prawn juveniles were randomly sampled and their individual initial length and weight were recorded. Security was provided to prevent poaching and predation by animals such as jackals.
Feed Formulation, Collection of Ingredients, and Commercial Feed
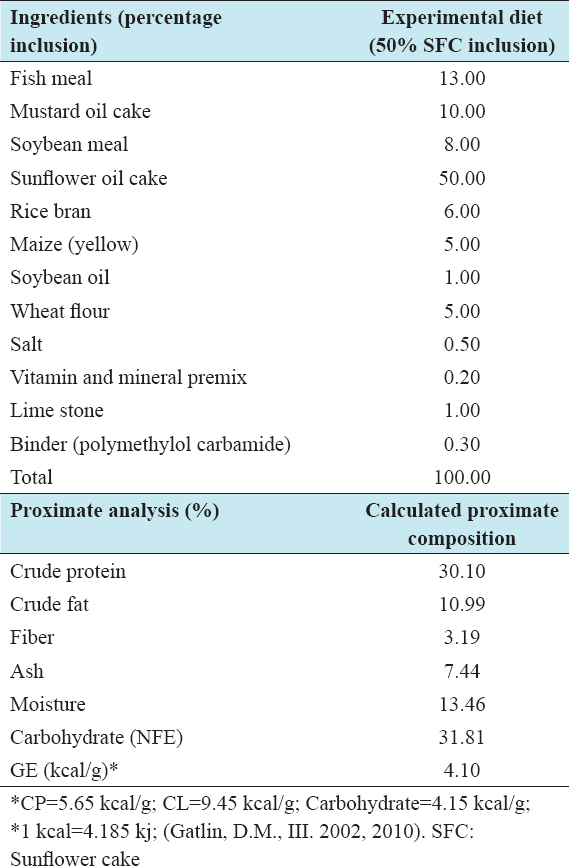
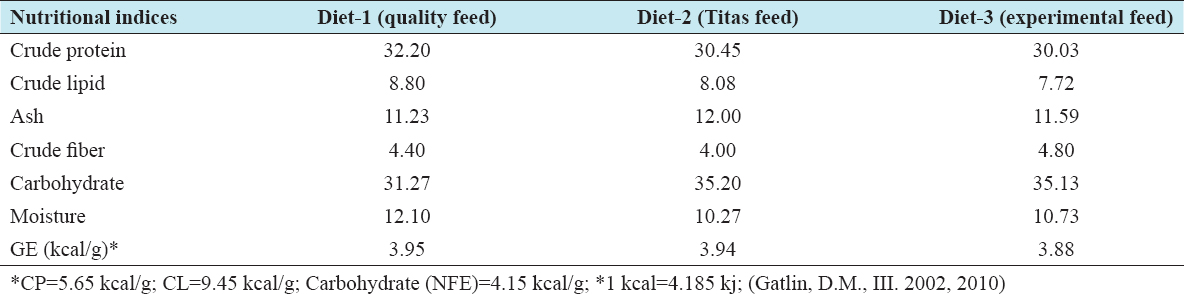
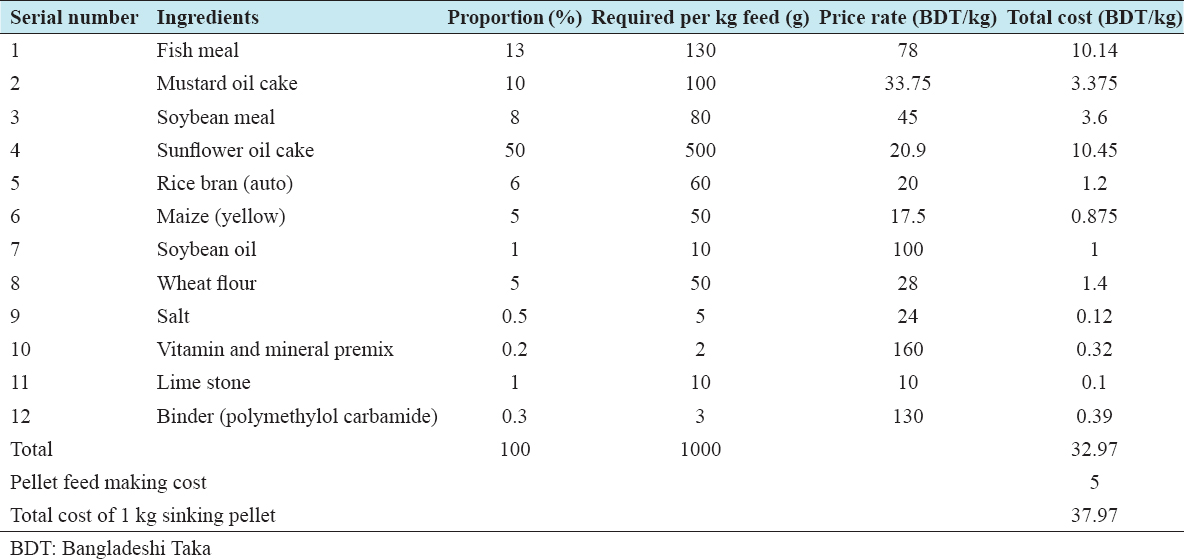
The experimental diet (W) was formulated based on the best performing Diet 4 of the previous experimental work in 2014, maintaining 50% inclusion of SFC, and around 30% crude protein.[7] To improve water stability of the experimental sinking pellet, a commercial binder (Polymethylol carbamide) was used at 0.30% inclusion, replacing an equivalent amount of rice bran. Ingredients for the experimental diet including SFC, fish meal, soybean meal, and mustard oil cake were purchased at one time so that nutritional composition could be maintained during feed formulation. In the experimental diet, fish meal, soybean meal, and mustard oil cake were partially substituted with 50% inclusion of SFC [Table 1]. Two commercially formulated feeds widely used by local farmers “Quality” and “Titas” feeds were purchased from a district dealer of the respective companies. The “experimental diet” and the two commercial feeds “Quality feed” and “Titas feed” were isonitrogenous (30.03–32.20% crude protein) and isocaloric (3.88–3.95 kcal). In Bangladesh, the feed companies do not use SFC in any of fish or prawn feed formulations. The ingredients commonly used are fishmeal, MBM, soybean meal, full-fat soybean, rice bran, maize bran, wheat bran, mustard oil cake, shrimp meal, vitamins, and minerals. To maintain equivalence, sinking pellets were chosen for the commercial feeds. Considering the size of the stocked juveniles, a starter feed was used for the initial 30 days and then grower feed for the rest of the period. The sizes of the experimental feed pellets were kept the same to that of the “Quality” and “Titas” brand feeds readily available in the market.
Table 1: Formulation and calculated nutritional indices of the experimental diet Diet-3 (W) for freshwater prawn (Macrobrachium rosenbergii)
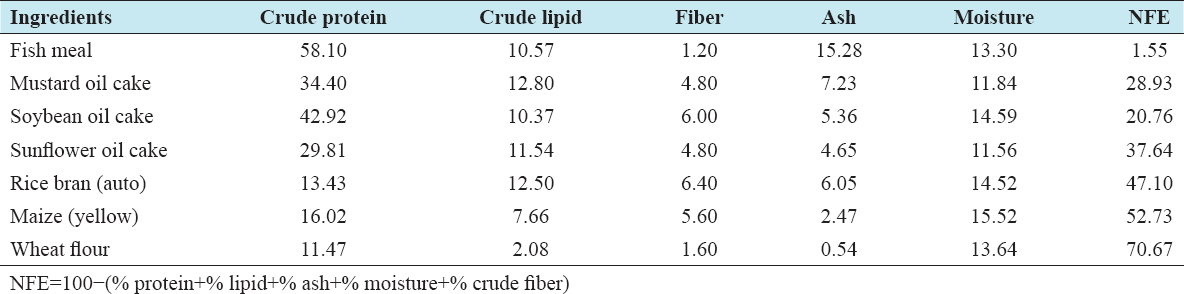
Whole dried chewa fish (Pseudapocryptes elongatus) were collected from a wholesale market in Khulna and crushed in a local feed mill to make a fish meal for the experimental trial. The suppliers reported that the dried chewa were collected from Dublar Char island of, Shoronkhola Upazila of Bagerhat District. Mustard oil cake, soybean meal, SFC, maize, and rice bran were collected from a local feed supplier in Khulna. Vitamin premix, binder, vegetable oil, limestone, and salt were purchased from a local shop [Table 2].
Table 2: Proximate composition of feed ingredients (percent basis)
Preparation of the Experimental Diet
Ingredients were crushed separately to powder in a mill and bagged. The required amounts of each feed ingredient were measured and mixed manually until a homogeneous mixture was obtained. Then, the mixture homogenized with vegetable oil and water at the rate of about 500 ml/100 kg ingredients and passed through a pelleting mill. After air cooling, the pellets were bagged in plastic bags and stored in a cool, dry place until used. The experimental diet was prepared as a sinking pellet in different diameters to suit the different stages of grow out and to depend on prawn size [Table 1].
Feeding Management
The prawns were fed to satiation twice per day, at around 7.00 am and 5.00 pm. Feeding was by broadcasting across the compartments. Predicted feed demand was calculated as 10% of prawn biomass per day initially, declining to 3% by harvest. The predicted feed amount was adjusted fortnightly after carrying out growth sampling. Feeding trays were set in each compartment to monitor feed intake by the animal and the feeding rate was adjusted depending on the time taken for the feed on the tray. Feeding was also suspended or reduced in the event of heavy rain, higher than usual air temperatures, etc., and details were recorded.
Growth Monitoring
Sampling was carried out fortnightly by catching around 10% of the stocked prawn population (20–25 prawns) from each compartment by cast net. Total length (cm) and weight (g) of individual prawn were recorded using a scale marked at 0.1 cm intervals and a digital balance sensitive to 0.1 g. A final sampling was carried out on the day before final harvest and sale of the prawns.
Water Quality Management
In contrast to the conditions during the previous experiment in 2014, heavy rainfall was experienced from the beginning of the experiment and continued medium to heavy during around 50% of the grow-out period. This contributed to the maintenance of an optimum water depth of around 90 cm.
Liming
To compensate for the frequent rainfall and maintain pH within the optimum range, liming was carried out on five occasions during the trial at a rate of 7.0–7.5 g/m2. Regular liming also helps in maintaining calcium levels in the water, supporting prawn growth. Mineralization of a newly molted prawn’s shell is affected by the availability of calcium, bicarbonate ion, and pH and can be improved through liming.[9]
Bottom Mud Raking
Raking of the pond bottom with a bamboo branch to stir the mud and help to release any trapped gases was carried out once a month.
Water Quality Monitoring
Water quality parameters including temperature (°C), transparency (cm), pH, salinity (ppt), dissolved oxygen (DO) (mg/L), alkalinity (mg/L), hardness (mg/L), and ammonia-nitrogen (mg/L) in each compartment were recorded at 10-day intervals. Water temperature was measured with a digital thermometer (brand: Conrad Electronic; model K102; capacity 200°C; readability 0.1°C, made in Germany) and water pH was measured with a pH meter (Brand: Hanna instruments S.R.L. com, Romania, model-HI 98107; range 0–14; resolution 0.1; accuracy ± 0.1; environment 0–50°C). Water transparency was measured using a locally made Secchi disk. DO was measured by DO meter (Brand: Hanna instruments S.R.L. com, Romania, model-PDO 520; range 0–20.0 mg/L×0.1 mg/L; temperature: 0–50°C). Ammonia-nitrogen (NH3-N) of water was determined by ammonia test kit (Brand: Hanna instruments S.R.L. com, Romania, Model HI 3824; Range 0.0–2.5 mg/L NH3-N; Analysis method: colorimetric [titration]). Total alkalinity of the grow out system was determined by test kit (brand: Hanna instruments S.R.L. com, Romania; Model HI 3811; range 0–100 mg/L CaCO3, 0–300 mg/L CaCO3; analysis method: Acid titration using phenolphthalein and bromophenol blue) and hardness was determined by test kit (Brand Hanna instruments S.R.L. com, Romania; Model HI 3812; range 0 – 30 mg/L CaCO3, 0–300 mg/L CaCO3; analysis method EDTA titration).
Harvesting and Postharvest Management
After 100 days, feeding was stopped the day before harvesting. On the day of harvesting, around 80% of the prawn biomass was harvested by cast net. Later, water was pumped out to ensure that all the remaining prawns could be collected by hand. Immediate after harvesting, the prawns were washed with clean water and put into buckets in an ice box. The prawns from each compartment were stored in separate buckets marked with the compartment code number.
Analysis of Feed Ingredients, Diets, and Carcass Composition
A sample of approximately 75 g of each feed ingredients used in the experimental diet “W” and of the two commercial feeds, Quality: “Q” and Titas: “T,” were collected and stored in well-marked plastic bags. 75 g sample of prawn from each compartment was collected at the beginning and end of the feeding trial and stored at −20°C for carcass composition analysis. The proximate composition analyses were carried out at the Department of Aquaculture Laboratory in the Faculty of Fisheries, Bangladesh Agricultural University, Mymensingh. The proximate composition analysis results of the ingredients, prepared diet, commercial feeds, prawn juveniles, and grown prawn samples are presented in Tables 2-4, respectively.
Table 3: Proximate composition of feeds (percentage fresh matter basis)
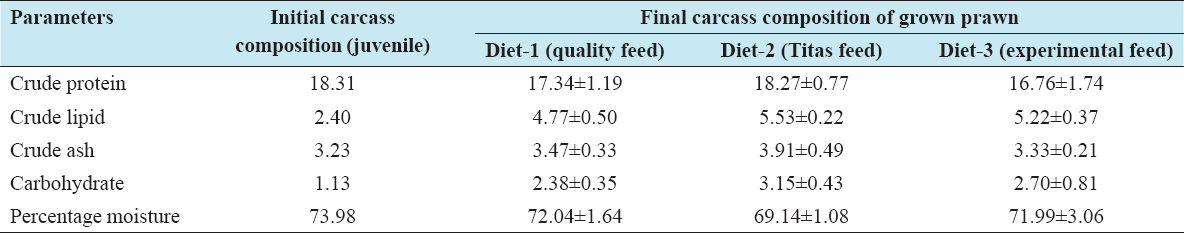
Table 4: Proximate carcass composition (percentage fresh matter basis) of whole juvenile and prawn fed different diets during a 100-day trial (mean±standard deviation)
The proximate composition of feed ingredients, feeds, and prawn carcasses was determined following the methods of AOAC.[10]
Growth Performance Parameters Analysis
The survival rate, weight gain, percent weight gain, specific growth rate (SGR), feed conversion ratio, and protein efficiency ratio were used to evaluate the growth performance of the prawns on the different diets, using the following formula e: [11]
(No correction was made for uneaten feed left on the pond bottom, so calculated values can be considered as apparent FCR)
Economic Evaluation
Economic analysis was based on the variable costs for inputs including prawn juveniles, feed, lime, pumping, and hired labor for repairs. Pond lease value and family labor were excluded from the calculation. The total cost of production was estimated based on local market prices during 2015 and expressed in Bangladeshi taka (BDT) (1 US$ = 78 BDT). The prawns produced were mainly sold to the local market. The total return from prawns was calculated as sales plus the amount consumed by the family multiplied by the sales price. Gross margin was estimated by subtracting the total variable costs from the total return. The benefit-cost ratio (BCR) was calculated using the following formula:
Statistical Analysis
Mean and standard deviations were calculated and expressed as “mean ± SD.” The significance of variations in the growth parameters and water quality was tested using one-way analysis of variance (ANOVA), which was followed by Duncan’s Multiple Range Test for significant values using SPSS software. Values were considered significant at 5% level of significance.
RESULTS
In all the treatments, the diets supplied were well accepted by the stocked prawns. The incidence of broken antenna disease experienced in 2014 was negligible in the current trial. However, from mid-September, the syndrome of edema at the carapace/tail joint and first segment of the tail was more prevalent and continued until harvest in the 1st week of October. Neighboring farmers also reported the same syndrome and believed that the continuous rainfall may have created favorable conditions for disease to spread due to rapid fluctuations in water quality. Apart from this, no abnormal prawns or any other major disease symptoms were observed during the experiment.
Physicochemical Parameters of Water
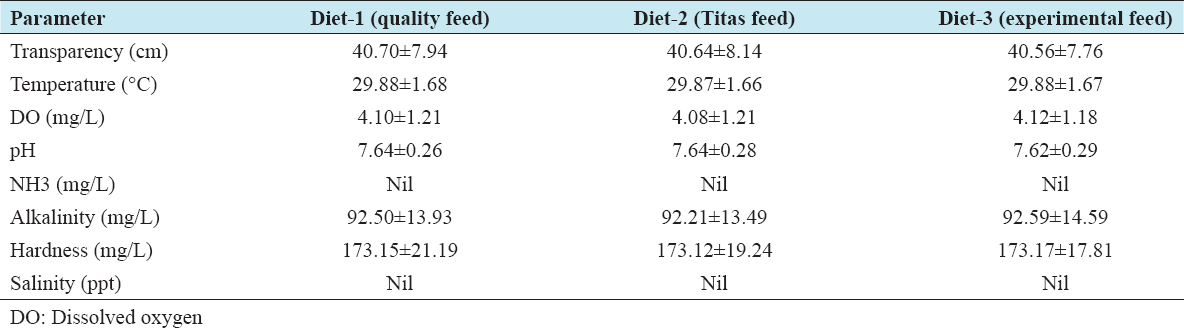
Overall mean values of water quality variables are presented in Table 5. Secchi disk readings were stable between 40.56 ± 7.76 and 40.70 ± 7.94 cm. The mean water temperature was observed within a range of 29.87 ± 1.66 to 29.88 ± 1.67°C and did not show any significant differences among the compartments throughout the study. The mean DO level was low and ranged from 4.08 ± 1.21 to 4.12 ± 1.18 mg/L. pH value was within 7.62 ± 0.29 to 7.64 ± 0.26 and ammonia-nitrogen throughout the grow out period was zero. Mean total alkalinity and hardness were almost similar among the treatments varying within a range of 92.21 ± 13.49 to 92.59 ± 14.59 mg/L and 173.12 ± 19.24 to 173.17 ± 17.81 mg/L, respectively. Zero salinity was observed throughout the study period [Table 5].
Table 5: Mean±standard deviation values of water quality parameters of the compartments of the commercial and experimental diets during the 100-day trial
Growth Performance in Response to Trial Feeds
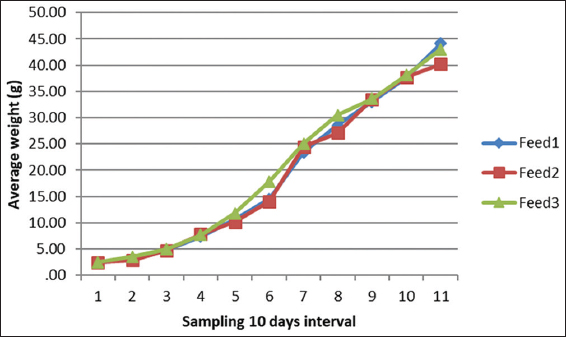
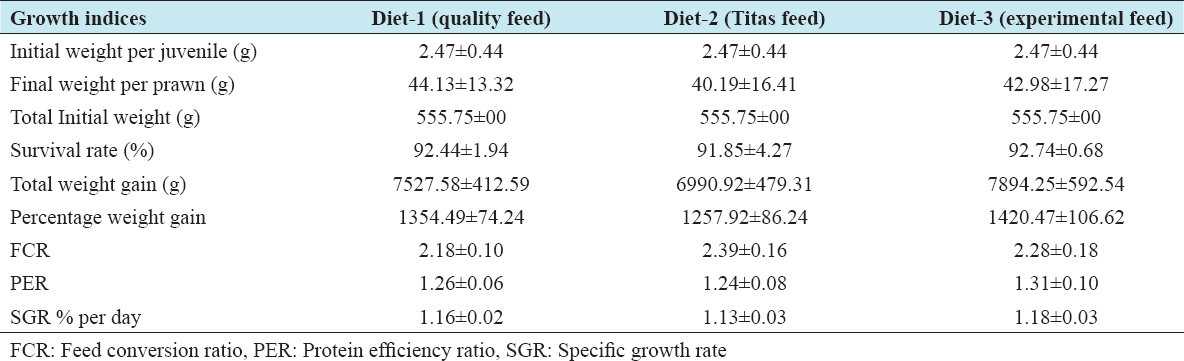
The growth performance of the diets used in the feeding trial for M. rosenbergii is given in Table 6. The survival rate of prawns varied from 91.85 ± 4.27 to 92.74 ± 0.68% among the different treatments. The highest mean survival rate was observed on Diet 3 followed by Diet 1 and Diet 2 but with no significant differences (P > 0.05). The average final weight of the prawn varied from 40.19 ± 16.41 g to 44.13 ± 13.32 g. The prawns fed on Diet 1 reached the highest average individual weight during the 100-day trial and those fed on Diet 2 reached the lowest [Figure 1 and Table 6]. However, after final harvesting, the highest total weight (8.450.00 ± 592.54 g) was achieved on Diet 3 followed by Diet 1 (8,083.33 ± 412.59 g) and the lowest was on Diet 2 (7,546.67 ± 479.31 g). The percent (%) weight gained also followed a similar trend, with the highest (1420%) for Diet 3, followed by Diet 2 and Diet 1. The differences of total weight (production) and percent weight gain were statistically not significant (P > 0.05). The specific growth rate (SGR% per day) for the diets varied between 1.13 ± 0.03 for Diet 2 as the lowest and 1.18 ± 0.03 for Diet 3 as the highest. Feed conversion ratio (FCR) of the diets is shown in Table 6 and varied from 2.18 ± 0.10 to 2.39 ± 0.16. The lowest FCR value was observed with Diet 1 and the highest with Diet 2. The differences in FCR between the diets were not statistically significant (P > 0.05). The protein efficiency ratio (PER) of the diets was found to be between 1.24 ± 0.08 and 1.31 ± 0.10. The lowest PER was obtained in Diet 2 and the highest in Diet 3 followed by Diet 1 (1.26 ± 0.06). There were no significant differences (P > 0.05) in PER between diets [Figure 1 and Table 6].
Figure 1: Variations in weight gain of prawns fed with three different diets
Table 6: Mean±standard deviation value of growth performance of Macrobrachium rosenbergii juvenile fed with different diets during a 100-day trial
Carcass Composition
Proximate analyses of carcass composition of prawn juveniles before the start of feeding and of the finally harvested prawn (M. rosenbergii) after feeding with two different commercial and one experimental diet for 100 days are presented in Table 4. No significant differences (P> 0.05) were observed in whole-body crude protein, crude lipid, crude ash, moisture, and dry matter contents among the treatments. The crude protein content of the stocked juveniles was found to be higher than that of the harvested prawn but with no statistical significance (P> 0.05). Higher carcass crude protein, crude lipid, crude ash, carbohydrate, and lower moisture content were observed on a fresh matter basis for the prawns fed Diet 2. Diet 1 was followed Diet 2 in case of crude protein and crude ash levels; however, Die 3 was followed Diet 2 in case of crude lipid, carbohydrate, and moisture.
Productivity and Economic Performance
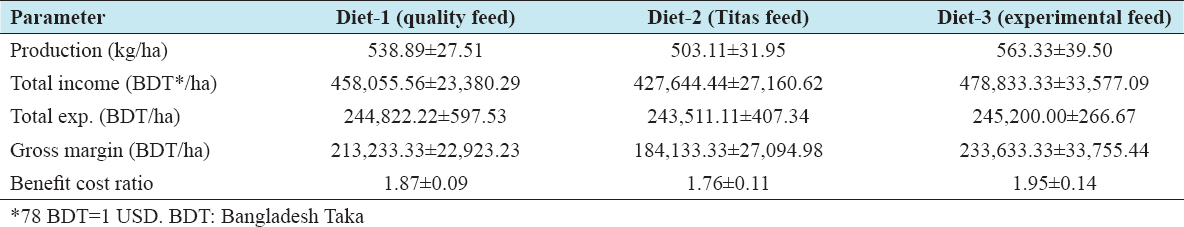
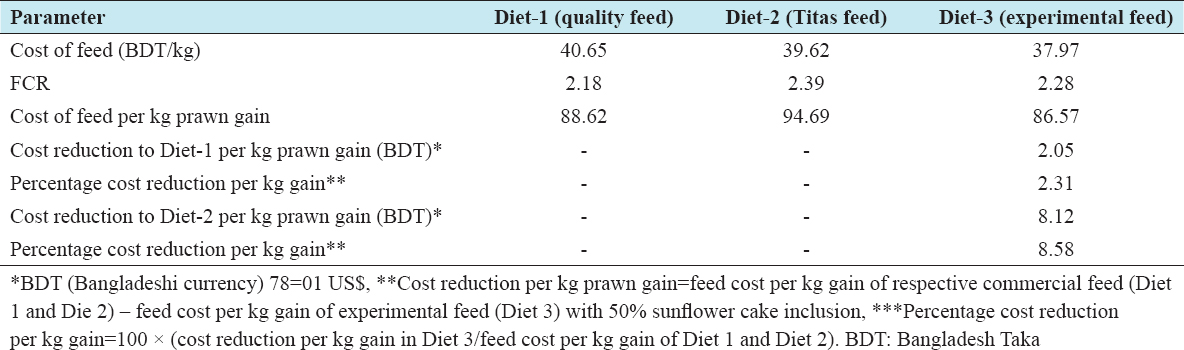
Productivity and income were estimated for the three different treatments with two commercial and one experimental diet for 100-day grow out. From Table 7, it is evident that the highest production (563.33 ± 39.50 kg/ha), income (BDT 478,833.33 ± 33,577.09/ha), and gross margin (BDT 233,633.33 ± 33,755.44/ha) were achieved with Diet 3 followed by Diet 1 and Diet 2. Among the harvested prawn, around 33% were male and 67% female; however, the contribution of males and females to the total weight was 43% and 57%, respectively. BCR also followed similar trend with the highest mean (1.95 ± 0.14) being for Diet 3 followed by Diet 1 and Diet-2. No significant differences (P > 0.05) were observed in BCR, productivity, income, or gross margin among the diets. The cost of 1 kg feed varied BDT of 40.65–BDT 37.97 was used in the treatments [Table 8a and b]. The lowest cost was for Diet 3 (BDT 37.97/kg) formulated with 50% inclusion of SFC, followed by Diet 1 and Diet 2. Combining FCR and the cost of different feeds, the cost of feed per kg prawn weight gain was the lowest for Diet 3 BDT 86.57, followed by Diet 1 and Diet 2 [Table 9]. The cost of production of 1 kg prawn was 8.58% lower (BDT 8.12) for Diet 3 than for Diet 2 [Tables 7 and 9].
Table 7: Performance in productivity and profitability of commercial and experimental feed for Macrobrachium rosenbergii during a 100-day trial (mean±standard deviation)
Table 8a: Unit cost of Diet-3 (formulated with 50% sunflower cake inclusion)
Table 8b: Unit cost of Commercial sinking pellet feed used in the trial
Table 9: Comparative economic efficiency of commercial and experimental feeds for Macrobrachium rosenbergii during a 100-day trial
DISCUSSION
Freshwater prawns are omnivorous and can digest a wide range of foods from both plant and animal sources.[12] Various cereal grains, oilseed cakes, brans, and several other animals and agro by-products have been reported to be used as ingredients in fish and prawn diets.[12-14] Soybean meal and SFC are probably the most promising alternative plant protein sources to replace a fish meal for prawn farming in Bangladesh. Although plant ingredients contain a variety of anti-nutritional substances such as protease inhibitors,[15] the use of SFC and soybean meal as a substitute for fish meal for many fish and shrimp has been reported.[16-19] Comparison of the biochemical makeup of SFC with fishmeal suggests some potential as a plant-based substitute for declining supplies of increasingly expensive fishmeal.[20] In freshwater prawn farming, farmers are found to use feeds from snail-meat to boil rice, rice bran, oil cake, and some cases commercial feeds. In most cases, farmers do not maintain any standard feeding practice but apply trial and error techniques.[21] However, there are very scarce data on growth performance of freshwater prawn (M. rosenbergii) with respect to the dietary inclusion levels of oil-extracted SFC. In Bangladesh, freshwater prawns which grow in earthen ponds usually enjoy favorable conditions of a subtropical environment and natural productivity. During this experiment, all three diets (two commercial and one experimental) benefited equally in this respect. No fertilizers were applied to enhance natural productivity, but lime was applied regularly to maintain water quality. Water quality parameters are the important variables influencing the biological performance of cultured aquatic species.
During the study period, all the physicochemical water parameters of the compartments used for the different diets were found to be within the acceptable range for freshwater prawn (M. rosenbergii) grow out.[9] Water transparency 40.56–40.70 cm, pH around 7.64, and DO 4.08–4.12 mg/L observed during the study were within the suitable ranges of 25–40 cm, 7.0–8.5, and 3–7 mg/L, respectively, recommended by New.[9] Water temperatures averaged 29.87–29.88°C were suitable for the growth of freshwater prawn.[9] However, the alkalinity (92.21–92.59 mg/L) and hardness (173 mg/L) recorded during the experiment were higher than the recommended 20–60 mg/L and 30–150 mg/L, respectively, by New.[9] The effects of water quality, as well as of planktonic food on the growth of the prawns, can be assumed to be similar in each treatment given the similar water quality parameters observed in all the compartments.
Prawn juveniles were stocked at a density of 15,000 number per ha and demonstrated similar survival rates (91.85 ± 4.27–92.74 ± 0.68) for all the treatments. Survival rate found in this study was higher than that of reported by[22] (64–78%) and,[23] stocked 10,000–30,000 PL/ha and recorded 68.23% survival. In the present experiment, juveniles were stocked instead of PLs, indicating the advantage of nursing PL to juvenile before stocking in grow out pond.
In the present study, the total final weight of prawn was not significantly (P > 0.05) different among the three feeds (commercial feeds “Quality” - Diet 1, “Titas” - Diet 2, and the experimental Diet 3). However, the highest average total final weight gain (7,894.25 ± 592.54 g/150 m2) of prawn was achieved with Diet 3, where 50% SFC was used, replacing some of both the animal and plant-based protein sources including fish meal, soybean cake, and mustard oil cake. Fish meal inclusion in Diet 3 was reduced to 13%. Diet 3 demonstrated slightly better SGR (1.18 ± 0.03) and PER (1.31 ± 0.10) than the commercial diets; however, better FCR (2.18 ± 0.10) was achieved with Diet 1 followed by Diet 3 (2.28 ± 0.18) and Diet 2 (2.39 ± 0.16). No significant differences (p>0.05) of those nutritional indices were found among the diets. All three diets in the study demonstrated better FCR than those reported by Gupta et al.,[22] or 2.99–3.18 found by Amaraweera et al.[24] using feed prepared with trash fish and various plant proteins and with a 34.26 ± 0.20% protein level. The lower FCR observed in the current study may be due to the lower stocking density and use of juvenile prawn. In addition, there is likely to be some growth benefit from the natural productivity of the earthen pond.[25] The SGR (% per day) of this study at 1.13–1.18% is similar to the findings of Muralisankar et al.,[26] but lower than those SGR reported by Gupta et al.[22] (1.84–2.24) and Amaraweera et al.[24] (2.03–2.08).
The different diets of the study exhibited better PER (1.24–1.31) than those reported by Muralisankar et al.[26] The highest growth performance including total and percent total weight gain of 7,894.25 g and 1420.47%, respectively, was with Diet 3 (the experimental feed) and the lowest growth performance was with Diet 2 (Titas feed). There were no significant differences (P > 0.05) of total and percent weight gained among treatments, although the growth rate of M. rosenbergii has been reported highly variable.[27] The result also concurs with the findings by Gupta et al.,[22] of relatively poor performance when prawns are fed with a diet containing fish meal as the major protein source compared to a diet containing both fish meal and plant protein such as soybean meal. In Diet 3, only 13% fish meal was used as an animal protein source and the remaining protein came from different plant sources including 50% SFC. The growth performance of prawn juveniles in the present study was found to be higher, compared to the findings by Hasanuzzaman et al.,[18] Hossain and Paul,[28] though they reported that a diet containing 30% protein using fish meal, MBM, oil cakes, and rice bran generated good growth of prawn juveniles reared for 90 days. The dissimilarity could be attributed to the differences in size of prawn juveniles stocked, natural productivity of the earthen pond, feed ingredients, and their nutritive value.
The final carcass composition of moisture, crude protein, and crude lipid of whole prawn fed with Diet 2 was higher than for the other two diets but not significantly different (P > 0.05). The lowest moisture content (69.14%) and highest crude protein (18.27%) and lipid (5.53%) were found with Diet 2. This was followed by Diet-1 and Diet-3 with crude protein (17.34% and 16.76%), respectively, and lipid (4.77% and 5.22%), respectively. An inverse relationship of crude protein and moisture was found in Diet 2 and Diet 1, with a slight deviation in the case of Diet 3. Gupta et al., also found identical carcass crude protein levels of 17.63% and 18.40% using feed containing 35.26% and 34.51% crude protein respectively.[22] In this study, a similar trend is evident with carcass protein of Diet 2, Diet 1, and Diet 3 of 18.27%, 17.34%, and 16.76%, respectively, compared to crude protein content of the diets of 30.45%, 32.20% and 30.03%, respectively. Those results demonstrated that diets made with differing protein sources such as fish meal, soybean meal, mustard oil cake, and SFC did not affect the carcass protein content of the prawn significantly (P > 0.05).[29]
Feedstuffs containing at least 20% crude protein are considered to be a protein supplement. SFC contains around 30% crude protein, rich in methionine and arginine but with a low level of lysine.[30,31] Lysine is readily available in planktonic feeds consumed by omnivores.[32] Considering the high palatability and low anti-nutritional factors of SFC (polyphenolic compounds only 1–3%), SFC has been used at up to 30% inclusion in fish feeds as an alternative plant protein source to fish meal with good results.[30] SFC can be incorporated at up to 5% in marine shrimp (Penaeus monodon) diet by replacing 20% of the fish meal, without compromising growth, and feed cost can be substantially reduced.[17] Similarly, up to 33% replacement with SFC in Atlantic salmon feed and 42% inclusion in rainbow trout feed had not found adverse effects.[33,34] Moreover, the fish meal used in this study was obtained from a locally available dried fish, with varying quality of crude protein due to species composition, storage conditions, and insect infestation during storage.[35]
The highest production per hectare in the 100-day trial, of 563.33 kg/ha, was obtained with Diet 3, while the range on the three diets was 503–563 kg/ha. All three diets gave higher production than the average annual yields of prawn as reported by Ahmed and Garnett[1] and Hasanuzzaman et al.[23] for the southwestern region of 425–440 kg/ha. These figures were for farmers stocking PL with a comparatively longer grow out period. The outcome of this study is also comparable with an average production of 432 kg/ha/yearin Bagerhat District of Bangladesh.[36] The higher production per hectare seen in the current study was probably due to the stocking of juveniles and a consistent feeding program. However, 676.5–721.9 kg/ha in 135 days grow-out, using a 35% crude protein feed production was obtained.[22] These data suggest that the results of the 100-day grow out trial are realistic and indicate the production potential of freshwater ghers in the region if good farming practice is followed.
The highest figures for total income, gross margin, and BCR per hectare from 100-day prawn farming were US$ 6139, US$ 2995, and 1.95, respectively, found in Diet 3. The gross margin is comparable to the findings of Ahmed,[3] who estimated annual net return as US$ 2162 per hectare. However, Diet 3 in the present study offered a feed cost representing a lower percentage (19.51%) of total operating expenditure compared to the 33% as reported by Ahmed.[3] Diet 3 with its 50% inclusion of SFC demonstrated an 8.58% lower feed cost/kg of prawn produced compared to Diet 2 (Titas, a regional commercial company feed), showing the potential of reducing traditional animal and plant protein ingredients. The low price of locally produced SFC also support Diet 3 for dietary cost savings.
In general, omnivorous species such as freshwater prawn require less dietary protein compared to carnivorous species.[37] M. rosenbergii is usually capable of digesting both plant and animal origin food and can utilize carbohydrate efficiently as a source of energy.[12] Most omnivorous species require a diet containing 25–35% crude protein.[27] Diets with 35–40% crude protein and protein/starch ratio of 1:1 demonstrated better growth and feed efficiency.[38] Diet 3 and the two commercial feeds used in the study fall within these guidelines and had near identical proximate composition. The utilization of dietary protein is mainly affected by its amino acid composition, the calorific content of the diet, digestibility of the protein, physiological state, age of the species, and water temperature.[25] The productivity of prawn is also closely related to the quality of seed and its availability at the right time.
CONCLUSION
The results of the study demonstrated that freshwater prawn (M. rosenbergii) feeds formulated with a high level (50%) inclusion of SFC can offer equally acceptable or potentially better performance than feeds made by reputable commercial companies and marketed in Bangladesh. SFC is locally available and offers equivalent nutritional quality while reducing the requirement for more expensive fish meal and soybean meal protein. Replacement of these more expensive ingredients with SFC has been demonstrated a positive impact on farm productivity and profitability. Feed containing 50% SFC and only 13% fishmeal inclusion demonstrated better survival rate, better SGR, better protein efficiency ratio, and lower feed conversion ratio compared to two commercial feeds. It is also noteworthy that fishmeal remains susceptible to unpredictable price increases, and has sustainability issues.[32] Due to the low price of SFC, Diet 3 also generated better gross margin and BCR compared to two commercial feeds. SFC is still little used as a prawn feed ingredient, but farming of sunflower in the southern region of Bangladesh could provide a cost-effective option for local prawn farmers. The study has demonstrated the competitiveness of the feed containing SFC with commercial feeds formulated without SFC inclusion and has indicated the possibility of inclusion rates of up to 50% while reducing fish meal to 13%. However, considering little work has been carried out to date with SFC as a prawn feed ingredient, further research is needed to find the optimum balance in feed formulations of SFC, other animal and plant protein sources.