INTRODUCTION
During the past decades, poultry industry has become the most expanding sector throughout the world. The intensive system of poultry production causes stress to the birds which hamper the immunity and productivity of chicken.[1] To solve this, commercial farms are widely using probiotics in animal and poultry ration. After using antibiotic, increased growth performance, lower mortality, and higher immune response in broilers are well evident.[2] However, regular use of antibiotics in the feed leads to the development of antibiotic resistant pathogenic bacteria,[3] thereby causing resistance to medicines, persistence of infections and retentions of antibiotic in different body parts of chicken which is recognized as a serious public health problem.[4] Moreover, wide usage of antibiotics leads to higher drug resistance to the pathogens in animal body which can be spread to human and causes detrimental effect. With growing concerns about antibiotic resistance and safety of livestock products for consumers, there is a great interest in finding alternatives to antibiotics for poultry production. Concerning the issues, priority has been given to produce substitutes for increasing the microbial growth either using useful microorganisms or non-digestible elements.
At present, the use of probiotics in the feed is very popular to improve growth and productive performances including body weight, daily weight gain, and dressing percentage.[5] Several factors are directly involved with the efficacy of probiotic in the diet of laying hens which include conformation of microbial species, supplementary dose, system and level of addition, composition of diet, age of birds, genotype, and environmental stress issues.[6] The supplementation of probiotics to laying hens has been found to improve feed efficiency, egg production, egg quality, nutrient digestibility, modulation of intestinal microflora, pathogen growth inhibition, and gut mucosal immunity.[7-9] Zhang et al.[10] reported that the dietary supplementation of 0.01% probiotic improved egg production and egg quality. Contrariwise, various opposing results were also described on the effects of supplementing probiotic on egg production and feed conversion efficiency.[11-13] Probiotic supplementation may also play an important role in altering the lipid metabolism and reduce the cholesterol content both in egg yolk[14] and serum.[15] The effectiveness of probiotic application may depend on factors such as microbial species composition (e.g., single or multi-strain), livability, supplemental administration dose, method and frequency of application, diet composition, bird age, and environmental stress factors. Therefore, the present experiment was conducted to evaluate the effects of different kinds of probiotics on the performance, blood properties, and egg quality and yolk fatty acid composition of laying hens.
MATERIALS AND METHODS
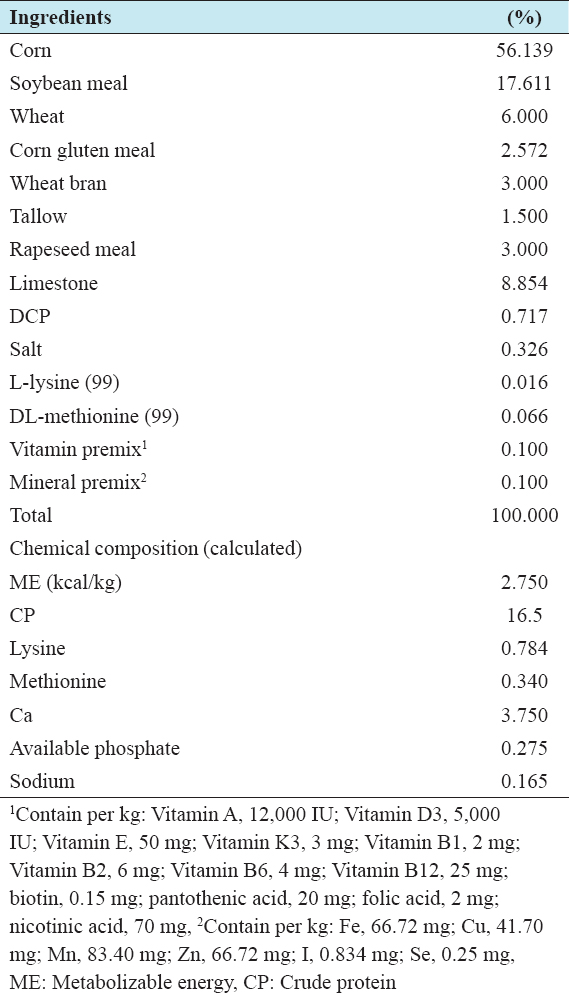
A total number of 360 Lohmann light laying hens were randomly divided into four groups with 5 replicates and 18 birds in each replicate pen. Treatment included (1) control (basal diet without probiotics), (2) inoculation of 0.1% P (probiotics) with basal diet (B), (3) inoculation of 0.1% probiotics with ginseng (PG) and (4) inoculation of 0.1% probiotics with sulfone (PS) in the diet, respectively. The temperature in the hen house was kept between 20 and 32°C. The light schedules were similar to the guidelines set in the Lohmann Commercial Management Guide. Birds had ad libitum access to feed (contain 2740 kcal/kg metabolizable energy and 16.2% crude protein) and water throughout the study (29–50 weeks). The basal diets are shown in Table 1. All other management of laying hens and experimental procedures were conducted in accordance with the Institutional Animal Care and Use Committee at Chonbuk National University, Korea.
Table 1: Ingredient (%) and composition of the experimental basal diets
Egg production, egg weight, and feed consumption were recorded on daily basis throughout the laying period. Egg mass was calculated by multiplying egg weight by egg production rate. The feed conversion ratio (FCR) was determined as a gram of feed consumed per gram of egg mass produced (g of feed/g of egg mass). At the termination of the trial, 30 eggs were arbitrarily collected from each treatment to determine the egg quality parameters. Breaking strength of the eggshell was assessed using an egg multitester device (QC-SPA, TSS, Cambridge, UK). The result was expressed as a unit of compression pressure applied to unit eggshell surface exterior area (kg/cm2). After that, each egg was weighed cautiously and then broken separately on a glass plate, and the color of egg shell, albumin height, Haugh unit, and yolk color was determined using egg quality equipment (QCM+ System, TSS).
At 50 weeks of age, 10 blood samples were collected from each group and allowed to clot for 2 h at room temperature and were centrifuged (1500 rpm for 15 min at 4°C), and the serum was collected and stored at −80°C until analysis. The samples were used to measure serum cholesterol, triglyceride (TG), high-density lipoprotein cholesterol, and low-density lipoprotein by the Konelab 20 Analyzer (Thermo Fisher Scientific, Vantaa, Finland) following the manufacturer’s guidelines.
Determination of fatty acid composition, 1 g of fresh egg yolk was weighed accurately in a glass tube and disintegrated in 4 mL of methanol-benzene (1:4, v/v). From that point, 200 mL of acetyl chloride was gradually included over a time of 1 min and tubes were firmly shut with Teflon-lined tops and subjected to methanolysis at 100°C for 1 h. Subsequently giving a cooling time of 15 min at room temperature, 2 mL of 6% K2CO3 was included the tubes followed by the expansion of 2 mL hexane for the vortex. Then, the tubes were jerked and centrifuged at 1700 g for 20 min. An aliquot of the upper stage hexane contained unsaturated fatty acid (UFA) methyl esters (FAME) was infused into the chromatograph. Unsaturated fats were chromatographed as methyl esters on a 30-m fused silica section having an inner distance across of 0.25 mm. The section was well-coated with 0.20 mm SupelcoTM 10. Investigation was performed on an Agilent Technologies 6890N, a gas chromatograph, outfitted with a fire ionization identifier. Helium was utilized as a bearer gas and nitrogen as a make-up gas. The split proportion was 100:1. The infusion port temperature in stove condition and the indicator was 240°C. The section temperature ascended in a stepwise way from 180°C up to 230°C at the rate of 3°C/min and afterward holds for 15 min. The fatty acids identified using a FAME standard and were expressed as percentage of total known FAME.
All data were analyzed by analysis of variance using the GLM procedure of SAS[16] with a predetermined significance level of P < 0.05. To compare means among the treatment groups, Duncan’s multiple range tests were used.[17]
RESULTS AND DISCUSSION
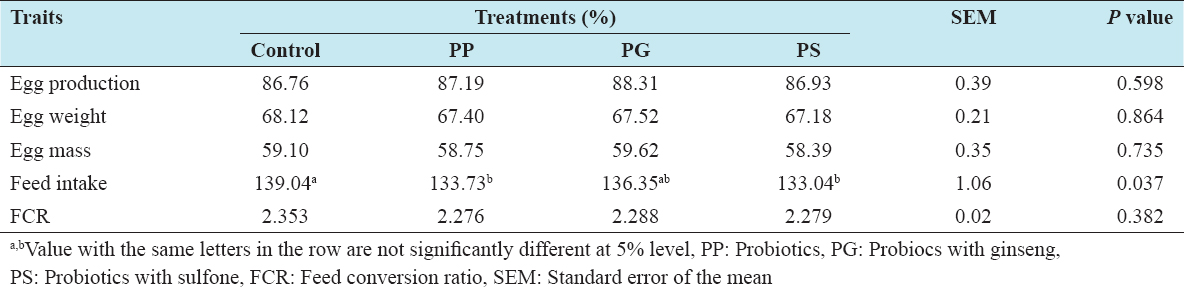
The effect of feeding probiotics on production performances of laying hens is presented in Table 2. The results indicated that 0.1% PG supplemented with basal diet had increased egg production than other three groups, but the effect did not reach to the significant level. In addition, chickens receiving diets containing probiotic and PS tended to have lower (P < 0.05) feed intake than the control group. Egg weight, egg mass, and FCR were not influenced by the supplementation of probiotics into the diet.
Table 2: Effect of feeding probiotics on the performance of laying hens
This result is consistent with previous studies which reported that supplying a probiotic mixture in the diet had no significant impact on egg production and egg quality.[18] In other investigations, Kurtoglu et al.[19] and Kalavathy et al.[15] reported that significant (P < 0.05) increases egg production after supplying a probiotic mixture in the diet. These differences might be due to the supplementation of different bacteria strains with different concentrations, the form of probiotics, and the ages of the hens.
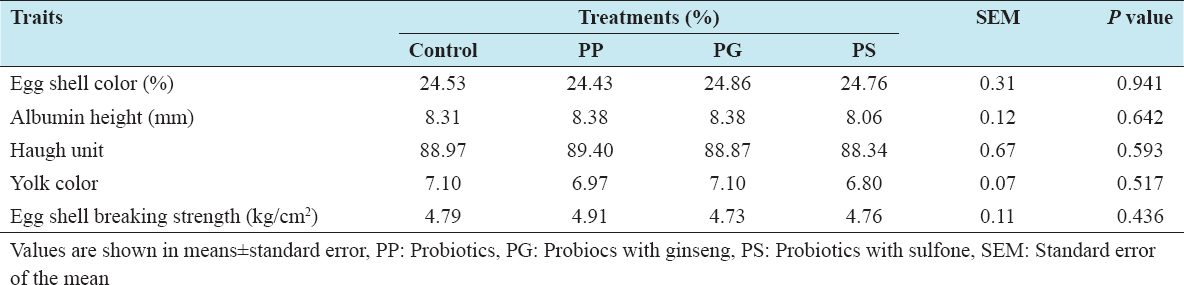
The egg quality data for the laying hens receiving the experimental diets are shown in Table 3. Laying hens receiving either PG or sulfone did not significant effects on egg shell color, albumen height, Haugh unit and yolk color of egg. On the other hand, eggshell breaking strength was not influenced by receiving PG or sulfone in the diet. In previous, Panda et al.[7] found that eggshell quality was improved using probiotic in laying hens. Recently, Ren et al.[20] stated that yolk height, yolk color, and Haugh units were not affected by probiotic treatments. In another experiment, Abdelqader et al.[21] mentioned that the development of eggshell parameters was connected to the promoting effect of probiotics on metabolic processes as well as calcium utilization. Therefore, this variation might be due to trace mineral content and utilization with microbial supplementation in the diet of laying hens.
Table 3: Effects of feeding probiotics on egg qualities
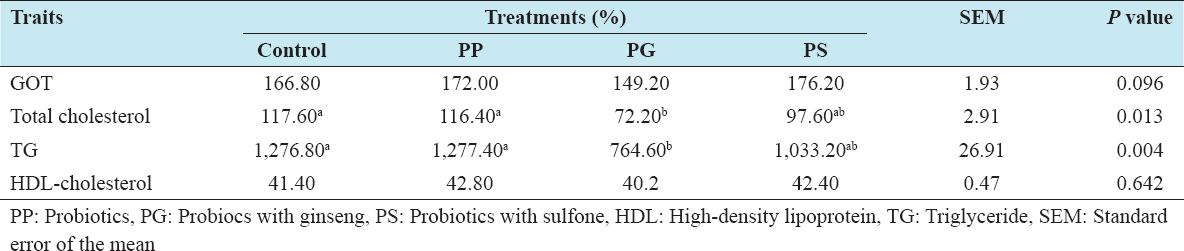
Analysis of blood serum parameters is shown in Table 4. Statistically significant difference in total cholesterol and TG content was observed in the group treated with PG. Serum total cholesterol and triglyceride content was reduced significantly by the addition of PG into the diet compared to control. The present results are consistent with the results of Mansoub[22] who reported that total cholesterol and TGs were decreased in the probiotic-treated group. A similar effect of probiotics on serum cholesterol level has been found in broilers.[23] Therefore, the reduction level of cholesterol could be due to cholesterol assimilation by the Lactobacillus cells[24] or to the co-precipitation of cholesterol with deconjugated bile salts, thereby decreasing pH level in the intestinal tract, which leads to reduce serum cholesterol.[25]
Table 4: Effects of feeding probiotics on blood composition of laying hens
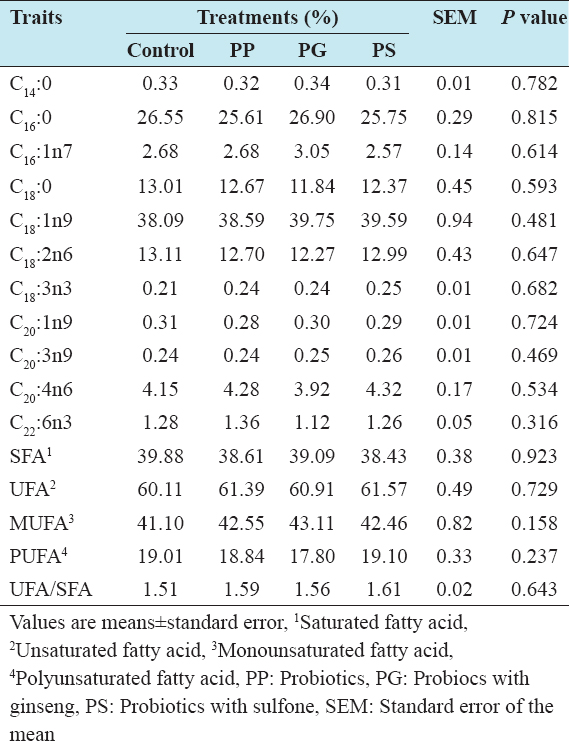
In the present experiment, the composition of fatty acid was not significantly influenced by the dietary treatments [Table 5]. Myristic acid (C14:0), Palmitic acid (C16:0), palmitoleic acid (C16:1n7), oleic acid (C18:1n9) and linolenic acid (C18:3n3) were found numerically higher in the group treated with probiotic and ginseng compared with control, probiotic, and probiotic with sulfone-treated group. Stearic acid (C18:0) and linoleic acid (C18:2n6) and (C20:1n9) were not affected in the dietary treatments.
Table 5: Effect of feeding probiotics on the fatty acid composition of eggs
The saturated fatty acid (SFA) was decreased and UFA was increased in the dietary groups compared with the control except PG. Similarly, monounsaturated fatty acid (MUFA) was higher and polyunsaturated fatty acid was lower in the dietary treatments than that of control group. Higher UFA and SFA ratio (UFA/SFA) was found in group treated with 0.1% PS probiotic in the diet. In previous study, Yalçın et al.[26] reported that Saccharomyces cerevisiae supplementation in diets for laying hens increased total SFA and the SFA/UFA ratio which corresponds with the present findings. On the other hand, Kalavathy et al.[15] found that hens fed diets with Lactobacillus culture had very little potential to modify the fatty acid composition of the egg yolk. In another experiment, Yalçın et al.[26] also stated that C18:1n9 and MUFA levels increased, and the other fatty acid parameters were not affected by yeast culture supplementation. This disparity was attributed due to the sample state between the present study and previous findings.
CONCLUSION
The present study demonstrated that 0.1% probiocs with ginseng showed positive effects on egg production and egg quality. It also decreased the serum cholesterol without affecting performance and egg quality of laying hens. Further follow-up studies should be conducted to investigate PG addition of >0.1% in laying hens diet.