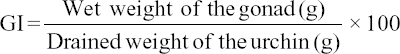INTRODUCTION
Salmacis sphaeroides (Echinodermata: Echinoidea: Temnopleuridae), commonly referred to as ball-like white sea urchin, occurs in the tropical Indo-West Pacific ocean where it can be found from China down to Australia.[1-3] It can also be found in the warm temperate regions including Johor States, between Malaysia and Singapore.[4-6] This species can occur at the range of depth between 0 m and 90 m, but it is mostly found in shallow waters, especially muddy sublittoral habitats with soft sediments (below the intertidal area).[7] They are often associated with macroalgae (seaweeds) and seagrass meadows or washed ashore and in coral reef habitats.[2,4] Various studies confirmed that S. sphaeroides is a generalist, feeding on a variety of seagrasses[8] and macroalgae,[9] sea pens, jellyfish, and a rather random plethora of experimentally introduced food items such as banana skins and salami.[10] They have also been observed attacking and preying on members of their own species.[11] It has been reported that this species has significant biological, ecological, aquacultural, pharmaceutical, and nutritional values.[5,6]
Sea urchins are found in oceans all over the world and greatly contribute to the food chains of marine environment by ingesting varieties of algae and invertebrates and also being consumed by mammals, fishes, crabs, sea stars and birds.[11] Gonads of sea urchin commonly known as “sea urchin roe” or “uni” are well accepted as a highly tasty food item in Asian, Mediterranean, and Western Hemisphere countries.[5,6,11-16] Either fresh or in the form of processed food, sea urchin gonad has long since been using as high delicacy and expensive food by the common Japanese peoples.[15-18] Despite S. sphaeroides has not yet been used as edible species in Malaysia, it has been found to serve as a delicacy food item in local seafood restaurants in Hong Kong.[19] Valuable bioactive compounds such as polyunsaturated fatty acids (PUPAs) and β-carotene are abundantly found in the gonads of sea urchin[20,21] and reported that the PUPAs, particularly eicosapentaenoic acid (EPA, C20:5) [n-3]) and docosahexaenoic acid (DHA C22:6) [n-3]), do have substantial protective effects on cardiac illnesses, arrhythmia, and cancer. Instead, the high amounts of arachidonic acid and EPA recently identified in S. sphaeroides[19] exhilarated the establishment of appropriate culture techniques of this high-valued sea urchin in captivity since PUPAs are essential for human nutrition.[22] Sea urchin gonad is also enriched with the first-class proteins and thus provide an excellent source of protein in human foods such as fish, meat, legumes, and beans.[11] However, in recent years, sea urchin fisheries have extended too largely that their population throughout the world have been depleted due to overfishing. These declining patterns clearly indicate the overexploitation of major fishery grounds and focus the necessity for proper conservation strategies, stock enhancement, fishery management, and aquaculture development to fill-up the gap between the supply and demand.[5,6,23-25]
The urchin research is quite new in Malaysia. However, a few studies on the population characteristics, distribution, feeding, breeding, and development of S. sphaeroides have recently been carried out,[1,5,6,26] no systematic studies have yet been conducted to optimize the juvenile and adult growth and production in rearing conditions. Hence, an effort has been carried out to establish a suitable aquaculture protocol of S. sphaeroides in a captive aqua-rearing condition.
MATERIALS AND METHODS
Broodstock Collection and Maintenance
Matured adults of the sea urchin, S. sphaeroides, weighing from 110 g to 180 g, were collected from Merambong shoal of Tanjung Kupang (01°34’ N; 103°60’ E), Johor, Malaysia, in July–October 2013, during which the urchins attain sexual maturity. Immediately after collection, the live sea urchins were transported to the Marine Biotechnology Laboratory, Institute of Bioscience, Universiti Putra Malaysia, where they were maintained in aerated aquaria before use for the experiments.
Induced Spawning and Fertilization
Gametes were collected from the sexually matured urchins by the intracoelomic injection with a 0.5 M concentration of potassium chloride solution. Spawned eggs were then shredded by placing the inverted female individuals on a transparent glass beaker filled with sterilized filtered seawater (FSW), while the concentrated sperms were pipetted off the genital pore from the male individuals. Fertilization of eggs was done using 10−5 diluted concentration of “dry” sperm.[27-29] Sperms were left for at least 10 min to ensure that all the eggs were encountered by spermatozoa during the fertilization process. Excess sperms and debris were then removed from the inseminated eggs by 3–4 consecutive washes with FSW.[5,30]
Larval Rearing
The incubation of fertilized eggs was followed in 500 ml glass beakers containing FSW at ambient temperature (25–26°C) until they formed into free-swimming blastula. They were then reared in 500-ml glass bottles containing SFSW on 10 rpm rotating rollers. Densities of larvae up to the four-armed pluteus stage were kept at 2–3 individuals/ml, using the protocols reported by Rahman et al.[27,29,31] When larvae attained four-armed pluteus stage, they were reared in 1000 ml glass bottles with a larval density of 1 individual/ml. The cultured unicellular diatom, Chaetoceros calcitrans, was supplemented as larval food at the rates of 5000, 10,000, and 15,000 cells/ml for four-, six-, and eight-armed pluteus stage, respectively, until attaining the metamorphic competence and settlement stage.[5,27]
Settlement Induction and Metamorphosis
After 30–34 days of larval rearing, when the matured larvae attained competent stage were then used for the settlement induction. Competence was judged by the evidence of large juvenile rudiments and a high metamorphosis rate.[30] Settlement induction and metamorphosis of competent larvae were done on coralline red algal extracts in plastic Petri dishes (9.0 cm × 3.0 cm) containing FSW. Density of larvae at this trail was maintained at one individual/2 ml FSW, following the method of Rahman et al.[30] and Rahman et al.[5] Transformation was proceeded by the absorption of larval arms and tissues, and the formation of complete juvenile structure with growing adult spines extended tube feet and well-developed pedicellaria, the entire event of which usually took place within 1-day post-settlement.[5]
Culturing of Juveniles and Adults
The newly metamorphosed juvenile urchins were cultured in small glass aquaria provided with continuous aerated FSW and the coralline red algae on the calcareous stones were supplied as diet.[27,29] The rearing seawater in aquaria was changed bimonthly with new FSW. This procedure was followed for a period of 3 months, during which time the juveniles reached to 9.0–10.0 mm in length. The 3-month-old juveniles with an average weight and length of 0.39 ± 0.05 g and 9.63 ± 0.31 mm, respectively, were then cultured in nine replicate glass aquaria (90 cm × 45 cm × 45 cm) and provided with aerated seawater in the grow-out culture unit of the Institute of Bioscience, Universiti Putra Malaysia. The stocking density was maintained at 30 juveniles per aquarium. The juveniles provided with red macroalga (Amphiroa fragilissima) as food were regarded as Treatment-1 (T1), brown macroalga (Sargassum polysystum) as Treatment-2 (T2), and green seagrass (Enhalus acoroides) as Treatment-3 (T3), respectively. They were fed ad libitum and the uneaten feed and feces were removed on regular basis. Seawater in each culturing aquaria was changed completely at every 2–3 months until the end of the grow-out experiments.
The physicochemical parameters of culturing waters were measured fortnightly at 09.00–09.30 h. Water temperature, salinity (ppt), dissolved oxygen (DO) (mg/l), and pH were measured instantly using a water quality analyzer (YSI Model 58, Yellow Springs Instruments, Ohio, USA) and ammonia nitrogen, nitrate nitrogen, nitrite nitrogen, and phosphate phosphorous by a standard HACH water analysis kit. Total alkalinity was determined using the established procedure.[32,33]
Growth performances in respects of length, weight, and health condition of the rearing urchins were monitored regularly. 10 individuals from each aquarium were measured at each 3 months of interval until they attained the adult stage. The culture was continued for 2 years and terminated on September 2015, within which the sea urchins achieved sexual maturity and also have sufficient mature gametes. Growth in terms of final length and weight, length and weight gain, specific growth rate (SGR), and daily growth rate (DGR), and survival was estimated the following standard methods. The SGR and DGR values were estimated the following Brown[34] and De Silva and Anderson,[35] respectively. Production of edible gonad was estimated the following Rahman et al.[5] while the gonad index (GI) was computed according to the formula[36,37,38] as given below:
Data Analyses
All percent data were arcsine transformed before used for statistical analyses. This transformation assisted to normalize the data and also reduced the heterogeneity in variances. A Bartlett’s test was used to analyze the homogeneity of variances.[39] When the variances were not significantly heterogeneous and did not have any major departures from normality, a one-way analysis of variance (ANOVA) was done followed by Tukey’s multiple comparison test, and the significance level was set at 0.05.
RESULTS
Physicochemical Parameters
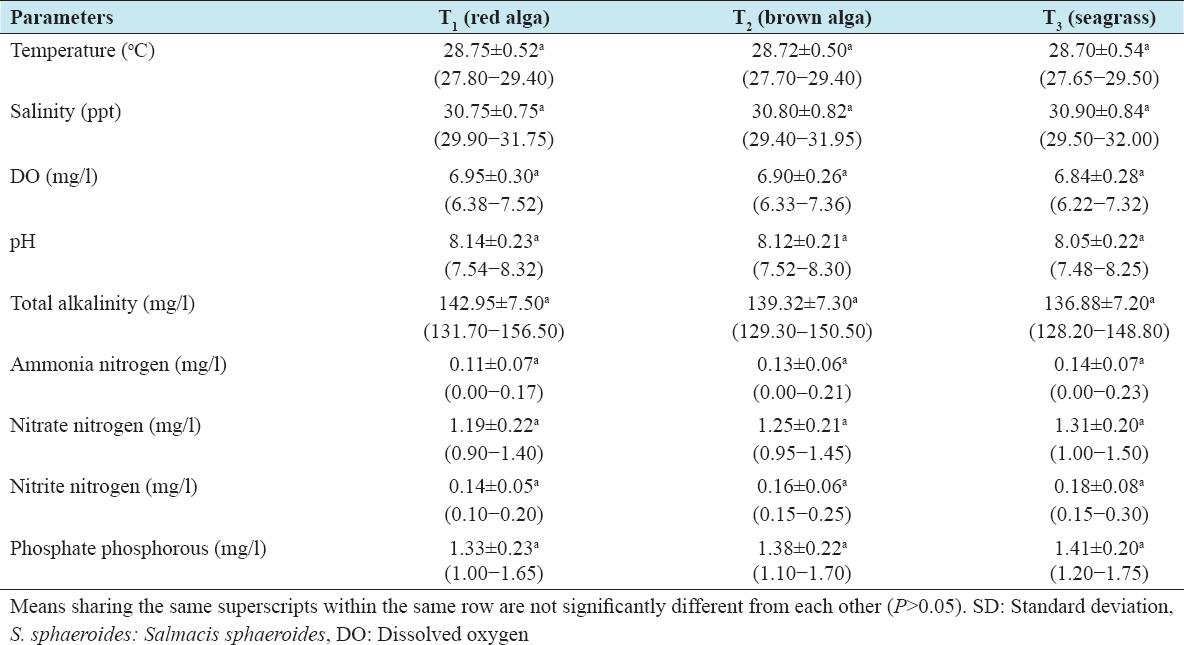
The mean and range values of water quality parameters in the rearing aquaria over the 2-year culture period of S. sphaeroides are summarized in Table 1. The mean values of temperature (oC), salinity (ppt), DO (mg/l), total alkalinity (mg/l), ammonia nitrogen (mg/l), nitrate nitrogen (mg/l), nitrite nitrogen (mg/l), and phosphate phosphorus (mg/l) did not show any significant differences (Tukey’s test, P > 0.05) among the treatments evaluated [Table 1].
Table 1: Mean±SD and range values of physicochemical parameters of seawater for the 2-year rearing period of S. sphaeroides in captive aqua-rearing condition
Growth and Production Performances
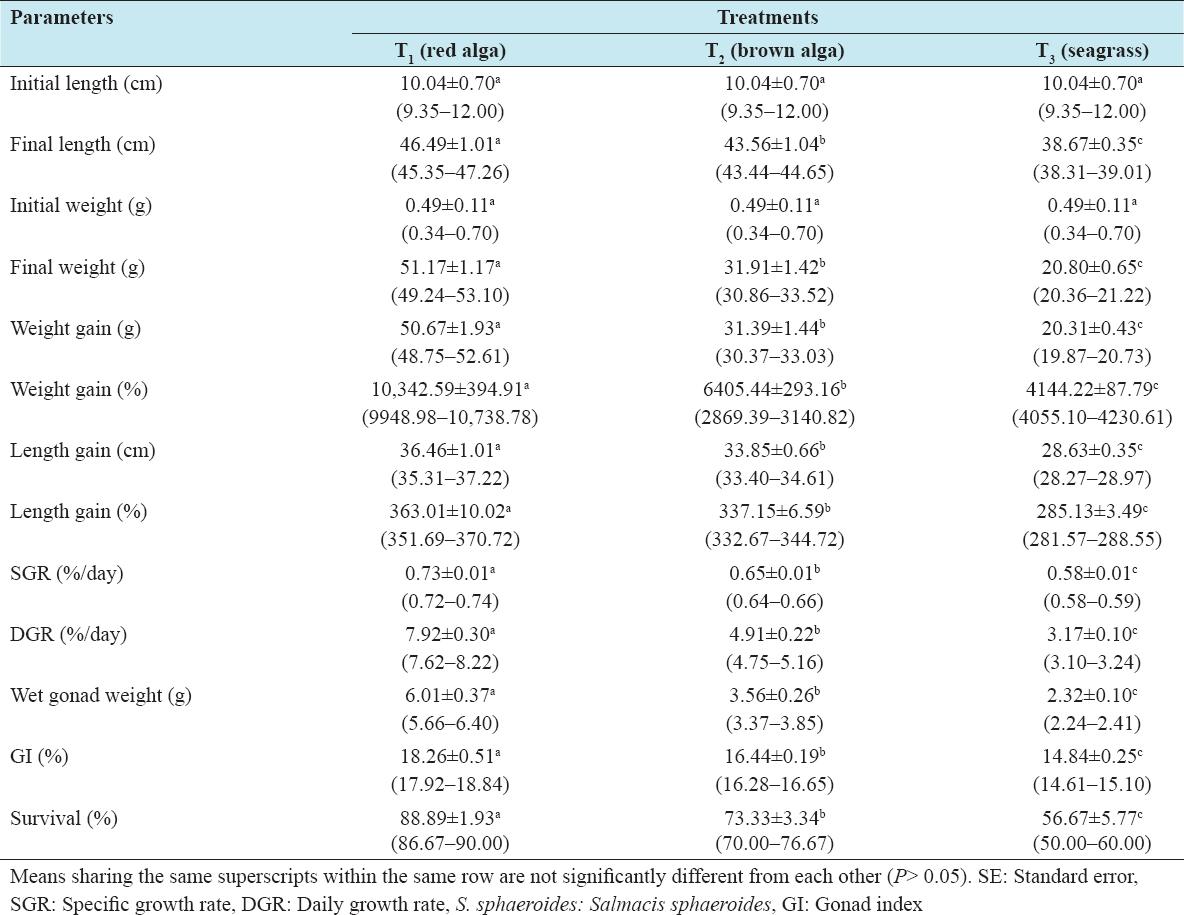
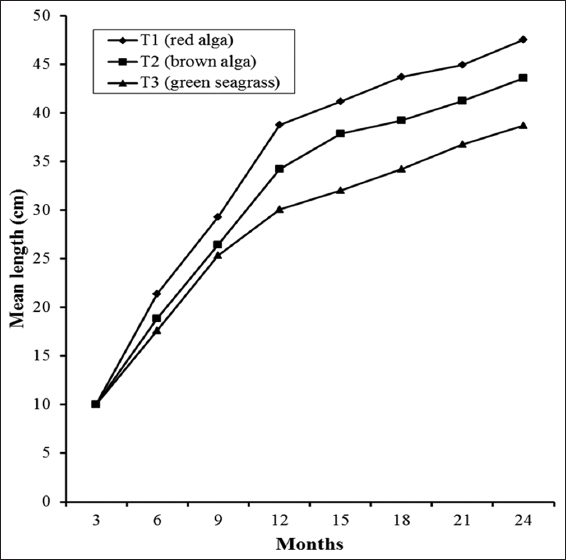
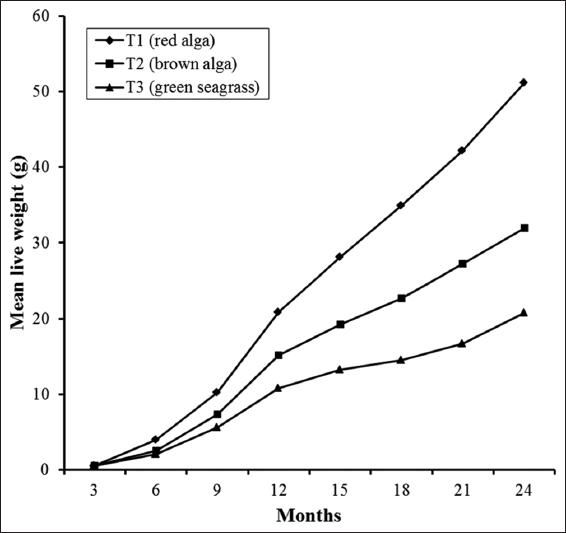
Detailed growth performances (such as final weight, final length, weight gain, length gain, SGR, DGR, gonad weight, and GI) and percent survival of S. sphaeroides at the termination of the 2-year culture period in the experimental treatments are presented in Table 2, while the growth trends in length and weight at each 3 months interval are depicted in Figures 1 and 2. The length and weight increment were the highest in T1 followed by that in T2 and the lowest in T3. The final mean length and weight of S. sphaeroides were significantly higher (Tukey’s test, P < 0.05) in T1 than the values obtained in T2 and T3. Similar trends were also found in weight and length gains. Percent weight gain and length gain were significantly highest in T1 and the lowest in T3 [Table 2]. Significantly higher (Tukey’s test, P < 0.05) SGR and DGR values were also obtained in T1 than those in T2 and T3 in this order.
Table 2: Comparison of growth and production parameters of S. sphaeroides fed with different algal feeds at the termination of a 2-year culture experiment. For each treatment, a total of 30 live urchins were measured for each parameter with 10 randomly selected individuals per replicate. All values indicate mean±SE and ranges in parentheses
Figure 1: Growth in respects of length increment in Salmacis sphaeroides fed with different macroalgae during the culture for 2 years
Figure 2: Growth in respects of live weight increment in Salmacis sphaeroides fed with different macroalgae during the culture for 2 years
Production of edible gonad was significantly higher (Tukey’s test, P < 0.05) in T1 than those produced in T2 and T3 [Table 2]. Similarly, the GI (percentage gonad weight in regard to the drained body weight) was significantly highest (Tukey’s test, P < 0.05) in T1 followed by T2 and the lowest in T3. However, the productions of edible gonad and GI in sea urchins fed with red alga (T1) showed an increment of 158.52% and 22.98% over seagrass (T2) and 69.07% and 11.03% over brown alga (T3) fed urchins, while it showed an increase of 52.97% and 10.76% over seagrass, respectively, when the urchins were fed with brown alga. Percentage values of mean survival in T1 (88.89%) did significantly higher (Tukey’s test, P < 0.05) than the survivals in T2 (73.33%) and T3 (56.67%), respectively [Table 2].
DISCUSSION
Coral reefs are considered as important habitats for most of the marine vertebrate and invertebrate organisms. Survival, reproduction, and development of marine organisms highly depend on the environmental factors such as water temperature, salinity, pH, and minerals.[40-44] Range values of water temperature (27.65–29.50°C) and salinity (29.40–32.00 ppt) in the experimental aquaria were within the appropriate level for sea urchin culture, which are in closer agreements with to the findings of Rahman et al.[27-29,45] The DO concentrations (6.22–7.52 mg/l) in our study were higher than that of Asia,[45] who recorded the DO levels ranged from 4.57 to 5.98 mg/l, while rearing collector sea urchin (Tripneustes gratilla) in glass aquaria and are within the suitable levels for grow-out culture in captivity. The pH values ranged from 7.52 to 8.32, agree well with the findings of Asia[45] and are in the good water quality levels for culturing of sea urchin under aquarium system. Besides these, the influences of other water quality parameters, namely total alkalinity, ammonia nitrogen, nitrate nitrogen, nitrate nitrogen, and phosphate phosphorous on body growth of sea urchins, have yet not been thoroughly examined. Nevertheless, we investigated these parameters for the 1st time in our present sea urchin culture trials and observed that all of them were within the suitable levels for sea urchins, as similar to those reported in various fish culture ponds.[46-53]
The growth parameters, survival rate, and edible gonad production of 2-year-old adults S. sphaeroides fed with red alga (A. fragilissima) were significantly higher (Tukey’s test, P < 0.05) in T1 than that fed with brown alga (S. polysystum) (T2) and green seagrass (E. acoroides) (T3). This may be because red alga was the preferred food in hastening the growth performances of S. sphaeroides than other algal foods. Likewise, coralline red algae were reported as one of the best algal diets in enhancing the growth performances, survival, and production of the adults of conspecific parents and the reciprocal hybrids among the different species of Echinometra spp. in Okinawa, Japan.[5,46,47] The results from our study are more or similar to the findings of Steinberg.[54,55] This contrasts with the observation of Sonnenholzner et al.[56] who found that the sea urchin (Strongylocentrotus purpuratus) fed on coralline red alga (Bossiella orbigniana) and the eelgrass (Phyllospadix scouleri) reduced their size and weight severely, and actually, these did not accelerate the growth of gonadal tissues for the rearing juvenile urchins as expected. Nevertheless, they also observed that a mixed diet comprising the aforementioned three algal species did perform well for the subadult of S. purpuratus than a single diet consisting of coralline algae (B. orbigniana) or eelgrass (P. scoulei) only.
In regard to the point of nutritional assessment, nitrogen has been considered as an essential part for the reproduction and growth of herbivores.[57] However, nitrogen (henceforth referred to as protein) is usually lower in all kinds of marine plants and appears to be the nutritional constituents, which best repeatedly impacts on the diet selections of herbivores.[56,58] For instance, the eelgrass and kelp contain similar amounts of proteins, lipids, and carbohydrates of 0.8–1.5%) and 40–45% (“dry” weight basis), respectively. Conversely, the coralline red alga, B. orbigniana, covers lesser amounts of proteins, fats, and carbohydrates of <3%, 0.5%, and 8–10%, respectively. Despite the fact that the eelgrass, P. scouleri was considered as a good source of protein similar to the brown kelp (E. menziesii), the highest growth rate was obtained in S. purpuratus when they were fed with kelp.[56] Even though we have not been able to estimate the proximate compositions of the algal plants used in this present study, it might not be overlooked that these plants can exhibit significant discrepancies in the contents of majority of its nutritional constituents that deserve more investigations.
In general, algae release extracellular organic matter, which may increase with stress.[28,59,60] It has also been reported that brown and green algae release higher amounts of polyphenols than do red algae[28] which was most probably accounted for the relatively much lower percent of metamorphosis and survival of juvenile urchins in treatments with these algae than did red algae.[28] Similarly, other studies have determined that the brown algae and green seagrass can seasonally produce several detergents such as condensed tannins and phenolic compounds, and thus, upsurge toxicity or decrease tastiness for herbivores, indicating the decline in nutritive value[61-63] and therefore, was perhaps the central key factors for restricting the ingestion of S. polysystum and E. acoroides by the juveniles and adults of S. sphaeroides in our experiments.
It is evident that the highest growth, gonad production and survival of S. sphaeroides were achieved from the sea urchins fed with red alga (T1) than those with brown alga (T2) and green seagrass (T3) in this order. This is the first successful approach to culture the high-valued tropical sea urchin, S. sphaeroides for 2 years in a captive aquaria-rearing condition. Due to the severe environmental perturbations and man-induced hazards in marine habitats, breeding, feeding, and living ground have been drastically degraded for this valuable sea urchin fishery in Malaysia. In these circumstances, the production of adequate quantities of sexually matured adult sea urchins using our current findings might be helpful toward the development of sustainable aquaculture and biodiversity conservation of S. sphaeroides. More researches are also acclaimed to search out more accurate seed production techniques, stocking densities, feeding regimes, and culture protocols of this high-valued sea urchin fishery to a greater extent.