INTRODUCTION
The World Health Organization reported that approximately 450 million people suffer from mental or behavioral disorders without proper treatment.[1] The onset of schizophrenia typically occurs in early adulthood between 15 and 35 years of age.[2] These challenges reflect possible increase in global bioburden of diseases from 12.3% to 15% by 2020. Some studies comparing the second-generation antipsychotics with typical antipsychotics failed to substantiate any major therapeutic advantage of the former.[3] Available medications can be very beneficial, but for most affected individuals, apathy, lack of volition, and social withdrawal (the “negative” symptoms) may be resistant to treatment even though the medications alleviate the hallucinations and aggressive behaviors, the “positive” symptoms.[4]
Cassia singueana a small tree shrub 1–6 m high with spreading rounded open crown 2 m in diameter. Trunk to 15 cm across, with dark gray, rough bark irregularly longitudinally fissured; slash light brown, yellow within. Stems of branchlets faintly longitudinally ridged to teret, young apice densely pubescent with curled white hairs interspersed among minute ones forming an underlayer, becoming sparsely pubescent and glabrous as bark develops. Leaves: Petiole and rachis 4–30 cm long. Stipules subulate, A ± 5 mm long, 3 mm wide, caduceus; petiole 1.5–5 cm long including basal pulvinus, petiole gland lacking, rachis channeled, with stalked, fusiform to elliptic, deciduous gland between each pair of leaflets, sometimes excepting the terminal leaflet. The ethyl acetate and ethanol root bark extract of C. singueana show promising antioxidant properties both in vivo and in vitro.[5] The methanol root extract of C. singueana was also reported to have hepatoprotective and hypolipidemic effects in rats.[6] C. singueana methanol leaf possessed antiulcer effect.[7] It was also reported by Adamu et al.[8] that C. singueana has antifungal activity.
MATERIALS AND METHODS
Collection of Plant Material and Preparation of Extract
The plant was collected in Dange Shuni Local Government Area of Sokoto in January 2018, Sokoto State, Nigeria. The plant was identified and authenticated in the Department of Pharmacognosy and Ethnopharmacy, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University, Sokoto, and was deposited in the herbarium. The leaves of C. singueana were shade dried. The dried leaves were pulverize to powder using wooden pestle and mortar and then 500 g of the dried powder was weighed and subjected to cold maceration for 72 h with 95% methanol as the solvent of extraction, the mixture was allowed to stand for 5 h and was then shaken periodically. Whatman filter paper was used for filtration of the mixture and the extract was concentrated on water bath at 40°C. The dried extract was transferred to air tide container for storage.
DRUGS
Apomorphine (Ranbaxy Laboratories, India, MFD: 10/13. EPD: 12/19), Diazepam (Neon Pharmaceutical Pvt., Ltd., MFD: 07/14. EPD: 08/20), Haloperidol (RPG Science Pharmaceutical Pvt., Ltd., MFD: 10/14. EPD: 01/22), methanol, and distilled water were used.
ANIMALS AND TREATMENT
Wistar rats of both sexes weighing (90–120 g) and mice (18–20 g) were used in this study. The animals were maintained in a cage well ventilated with access to food and water ad libitum. All animal experimental protocols were conducted in compliance with human-animal care standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Diazepam-induced Sleep
The method described by Beretz et al.[9] and modified by Rakotonirina et al.[10] was adopted. Briefly, 24 mice of either sex were assigned into four groups, each containing six mice. The first group received 10 ml/kg distilled water orally. The second, third, and fourth group received 200 mg/kg, 400 mg/kg, and 800 mg/kg orally of the C. singueana extract, respectively. 1 h post-treatment, all the mice were treated with diazepam 30 mg/kg orally, the animals were observed and onset and duration of sleep were recorded. Loss of righting reflex is considered as sleep onset and time interval between loss righting reflex and recovery to straightening were recorded as the duration of sleep.
Test for Exploratory Behavior in Mice (Hole-oard Test)
The method described by File[11] was adopted. Briefly, the apparatus includes white painted wooden board (60 cm × 30 cm) with 16 evenly spaced holes (1 cm diameter × 2 cm depth). 30 mice were randomly divided into five groups each containing six mice. The mice in the first group serve as the control which received 10 ml/kg distilled water orally. The mice in the second, third, and fourth group received 200, 400, and 800 mg/kg orally of the extract, respectively, while the mice in the fifth group received 1 mg diazepam per kg body weight orally. 1 h post-treatment, each mouse was placed at a corner of the board and the number of head dips in the hole was counted using a tally counter during 5 min.[12]
The Mice Beam Walking Assay for Motor Incoordination
The method previously described by Stanley et al.[13] was adopted for this study. Briefly, adult mice were trained to walk from a start platform along a ruler (80 cm long and 3 cm wide) elevated 30 cm above the bench by metal support to a goal box. Three trials were performed for each mouse and were designed such that the mouse tested would be aware that there was a goal box that could be reached. The mice that successfully walked along the ruler were randomly grouped into five groups each containing six mice. The first group received 10 ml/kg distilled water p.o. The second, third, and the fourth groups received 200, 400, and 800 mg/kg of the extract p.o, respectively. The fifth group received diazepam (1 mg/kg body weight, p.o).
Apomorphine-induced Stereotypic Climbing
The method described by Protais et al.[14] and modified by Costall and Naylor[15] was adopted for the study. Briefly, 24 adult mice were divided randomly into four groups each containing six mice. The first group received 10 ml/kg distilled water p.o. The second, third, and the fourth groups received 200, 400, and 800 mg//kg, respectively, p.o. 1 h post-treatment, all mice were treated with apomorphine (30 mg/kg body weight, subcutaneously). Each mouse was placed singly in a wire mesh stick cage and the climbing behavior was observed at 10, 20, and 30 min interval after apomorphine administration and scored as follows: 0 = four paws on the floor; 1 = fore feet holding the vertical bars; and 2 = four feet holding the vertical bars.
Catalepsy Test
The catalepsy procedure has been described previously.[16] Animals will first be examined in the crossed-leg position test and immediately thereafter in the bar test. In the crossed-leg position test, the hind limbs are placed over the ipsilateral forelimbs and the time during which an animal remained in this position was determined up to a maximum of 30 s. In the bar test, the forelimbs were placed on a horizontal, cylindrical metal bar (diameter 1.25 cm and height 10 cm), and the time during which both forelimbs remained on the bar was determined up to a maximum of 30 s. The bar test was repeated 3 and 6 min later and the mean of three trials was used for data analysis. Animals were returned to their cage between tests. Six animals were tested in each group. Positive control: Haloperidol, negative control: Distilled water, and test groups received 200, 400, and 800 mg/kg of the extract.
Data Analysis
Results were expressed as mean ± standard error of mean (SEM) and percentages. Data analysis was performed using SPSS statistical software (version 2.0). Comparison between groups was made using analysis of variance and Kruskal–Wallis test where necessary. When statistical difference was obtained, a post hoc Dunnett’s test was performed for multiple comparisons depending on nature of data. Values of P < 0.05 were considered statistically significant.
RESULTS
Effect of C. singueana Methanol Leaf Extract in Diazepam-induced Sleep in Mice
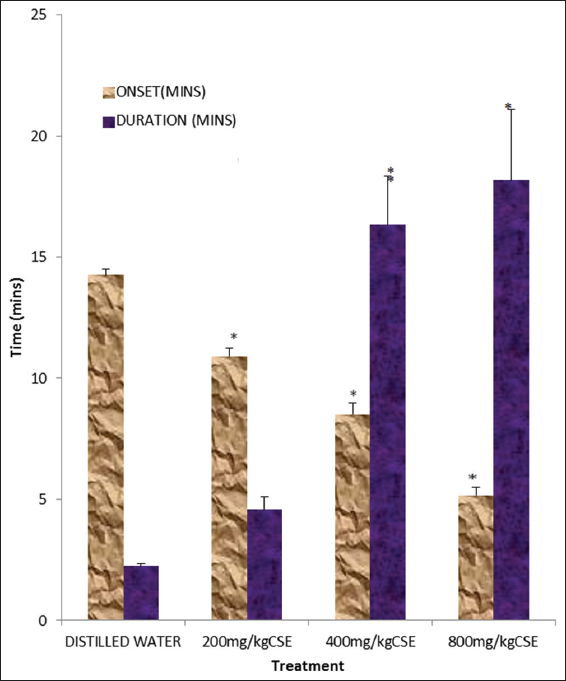
The methanol leaf extract of C. singueana significantly (P < 0.05) and dose dependently decreased the onset and increased the duration of sleep induced by diazepam [Figure 1].
Figure 1: Effect of methanol leaf extract of Cassia singueana on diazepam-induced sleep in mice. CSE: C. singueana methanol leaf extract. Data are presented as mean ± standard error of mean; *P < 0.05, n = 6, Dunnett’s post hoc test
Effect of Methanol Leaf Extract of C. singueana on Beam Walking Assay for Motor Coordination in Mice
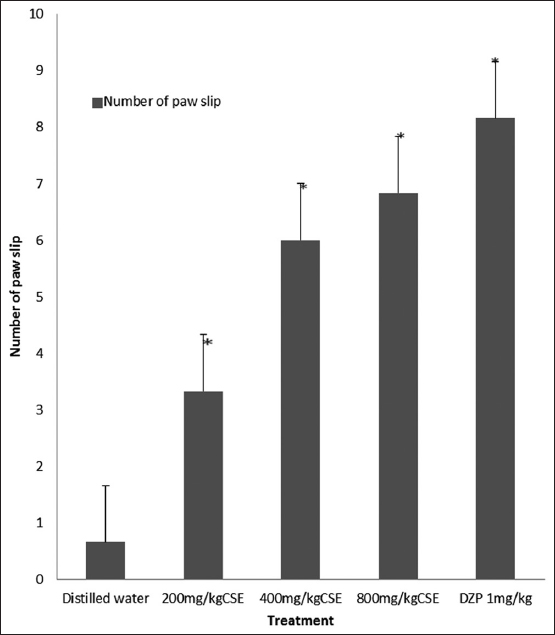
C. singueana methanol leaf extract significantly (P < 0.05) increased the number of foot slip by mice in the beam walk assay; increased foot slip indicates motor coordination deficit [Figure 2].
Figure 2: Effect of methanol leaf extract of Cassia singueana on beam walking assay for motor coordination in mice. CSE: C. singueana methanol leaf extract. Data are presented as mean ± standard error of mean; *P < 0.05, n = 6, Dunnett’s post hoc test
Effect of Methanol Leaf Extract of C. singueana on Hole-board Test for Exploratory Behavior in Mice
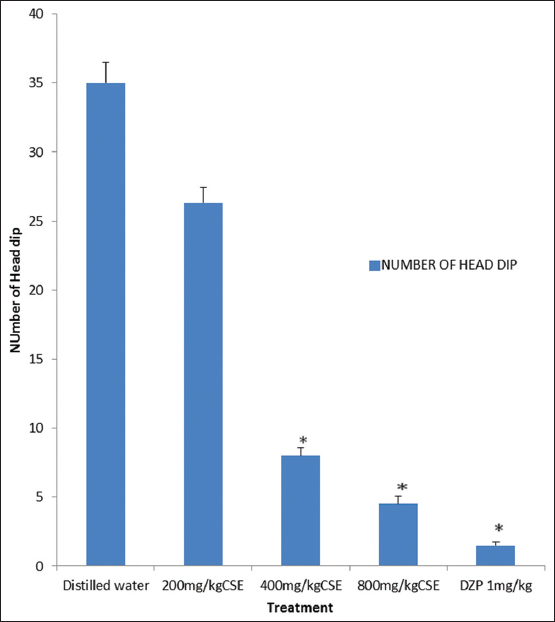
C. singueana methanol leaf extract significantly (P < 0.05) and dose dependently decreased the number of head dips in the hole-board test, indicating decreased in exploratory behavior [Figure 3].
Figure 3: Effect of methanol leaf extract of Cassia singueana on hole-board test for exploratory behavior in mice. DZP: Diazepam, CSE: C. singueana methanol leaf extract. Data are presented as mean ± standard error of mean; *P < 0.05, n = 6, Dunnett’s post hoc test
Effect of Methanol Leaf Extract of C. singueana on Apomorphine-induced Stereotypic Climbing in Mice
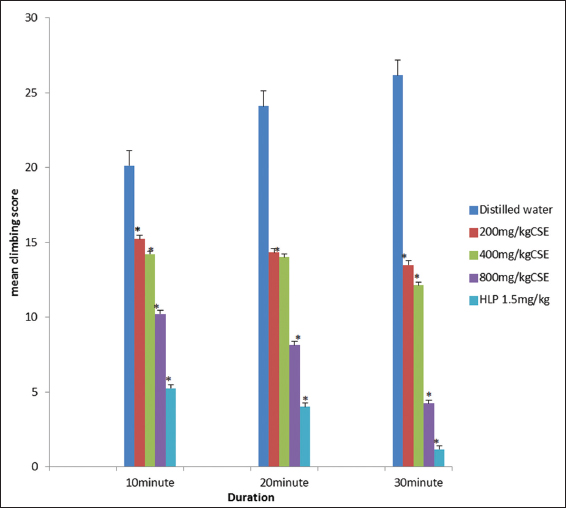
C. singueana methanol leaf extract significantly (P < 0.05) decreased the stereotypic climbing induced by apomorphine [Figure 4].
Figure 4: Effect of methanol leaf extract of Cassia singueana on apomorphine-induced stereotypic climbing in mice. HLP: Haloperidol, CSE: C. singueana methanol leaf extract. Data are presented as mean ± standard error of mean; *P < 0.05, n = 6, Dunnett’s post hoc test
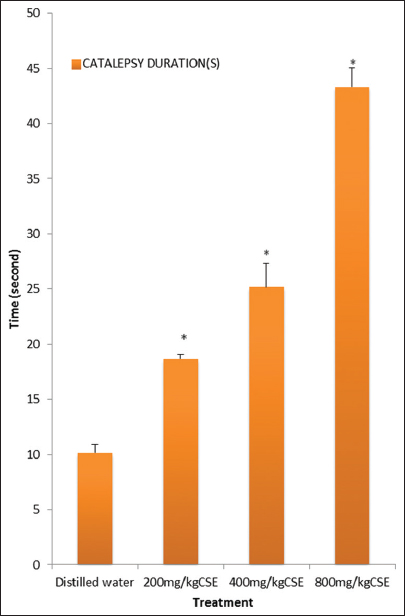
Effect of Methanol Leaf Extract of C. singueana on Catalepsy Duration in Mice
C. singueana methanol leaf extract significantly (P < 0.05) increased the duration of catalepsy induced by haloperidol [Figure 5].
Figure 5: Effect of methanol leaf extract of Cassia singueana on catalepsy duration in mice. CSE: C. singueana methanol leaf extract. Data are presented as mean ± standard error of mean; *P < 0.05, n = 6, Dunnett’s post hoc test
DISCUSSION
Plants chemical constituents constitute an indispensable part of medicinal plants and are responsible for their numerous bioactivities C. singueana methanol leaf extract significantly reduced the onset and prolonged the duration of sleep induced by diazepam. This action suggests that the C. singueana methanol leaf extract may possess sedating properties. It has been reported that saponins show significant sedative activity when tested in similar models.[17] Alkaloids are the most important secondary metabolites in many plants that are held responsible for their sedative and anxiolytic action.[18]
This sedative-hypnotic property could be related to the presence of triterpenes. It was previously reported that triterpenes activate benzodiazepines, barbiturates, and/or gamma-aminobutyric acid (GABA) receptors in the GABAA receptor complex.[19]
C. singueana methanol leaf extract significantly increased the number of foot slip by mice in the beam walk assay; increased foot slip indicates motor coordination deficit.[13] A similar finding was also reported by Magaji et al.[20] who noticed a central nervous system depressant effect of a plant by its virtue to cause motor incoordination using beam walk assay.[20] While using a different plant to assay its neurobehavioral effects Magaji et al.[21] also reported no significantly increase in number of foot slips in the beam walking assay, suggesting that the extract used does not possess significant motor coordination deficit and this finding suggests the plant may be devoid of central nervous system depression.[21]
C. singueana methanol leaf extract significantly and dose dependently decreased the number of head dips in the hole-board test, indicating decreased in exploratory behavior. As reported by Wadenberg,[22] the hole-board experiment is a measure of exploratory behavior in animals. A decrease in exploratory behavior indicates central nervous system depressant effect[30] Similarly, Magaji et al.[20] reported that a different plant possesses central nervous system depressant effect by decreasing exploratory behavior of the experimental animals. Furthermore, Adzu has reported a different plant with central nervous system depressant effect to decrease a number of head dips in hole-board test.
C. singueana methanol leaf extract significantly decreased the stereotypic climbing induced by apomorphine. The ability of drug to inhibit apomorphine-induced climbing behavior in mice has been correlated with neuroleptic activity. Inhibition of apomorphine-induced climbing in mouse is suggestive of D1 and D2 receptor blockade. The ability of the extract to antagonize apomorphine-induced climbing behavior supports the hypotheses of central nervous system activity which might be related to antidopaminergic actions on the limbic system as suggested by Anca et al.[23] and Morais et al.[24] A similar finding has been reported by Magaji et al.[20] Furthermore, Sotoing[25] reported a decrease in climbing behavior induced by apomorphine after oral administration of a neuroleptic extract. Amoateng et al. have also observed a significant reduction in the frequencies of apomorphine-induced rearing and cage climbing in mice after oral administration of an extract with possible antipsychotic activity.[26]
C. singueana methanol leaf extract significantly increased the duration of catalepsy induced by haloperidol. Increase or prolongation of haloperidol-induced catalepsy is correlated with neuroleptic activity and possible extrapyramidal side effect. Catalepsy in laboratory animals is defined as a failure to correct an externally imposed posture, and the test entails measuring the latency for the animal to remove itself from the unfamiliar and uncomfortable position. In mice, induction of catalepsy with haloperidol and other selective D2 receptor antagonist shows marked affinity for D2 receptor and predictive value for the identification of drugs liable to induce extrapyramidal side effects in humans. Extrapyramidal side effects are a major side effect that is known to account for the discontinuation of antipsychotic drug use in patients.[28] Ultimately, the current research for new antipsychotic drug discovery is focused on agents with minimal or no extrapyramidal side effects in addition to clinical efficacy.[27,28]
Our result reveals that the extract of C. singueana contains pharmacologically active substance(s) that might be acting centrally through the inhibition of dopaminergic pathway or a system linked to this pathway to mediate the prolongation of the cataleptic duration supported by Pandhare et al.[29]
CONCLUSION
From the data obtained in this research, it can be concluded that, Cassia singueana methanol leaf extrac may contain phytochemicals with antischizophrenic and central nervous system depressant effect.