INTRODUCTION
Arsenic present in the environment and humans all over the world is usually exposed to lesser amounts, mostly through food, water, and air. However, the presence of elevated levels of arsenic in groundwater, the main source of drinking water in many countries around the world, has drawn the attention of the scientific community. Groundwater, free from pathogenic microorganisms and available in adequate quantity through tube wells sunk in shallow aquifers in the floodplains, provides low-cost drinking water to scattered rural populations. Unfortunately, millions are exposed to high levels of inorganic arsenic through drinking this water. It has become a major public health problem in many countries in South and East Asia and a great burden on water supply authorities. Treatment of arsenic contamination of water, in contrast to that of many other impurities, is difficult, particularly for rural households supplied with scattered hand pump tube wells. In developing countries like Bangladesh, India, Pakistan, and Myanmar, the high prevalence of contamination, the isolation and poverty of the rural population, and the high cost and complexity of arsenic removal systems have imposed a programmatic and policy challenge on an unprecedented scale. Source substitution is often considered more feasible than arsenic removal. The use of alternative sources requires a major technological shift in the water supply. Treatment of arsenic-contaminated water for the removal of arsenic to an acceptable level is one of the options for safe water supply. Since the detection of arsenic in groundwater, a lot of efforts have been mobilized for treatment of arsenic-contaminated water to make it safe for drinking. During the past few years, many arsenic detection and test methods and small-scale arsenic removal technologies have been developed, field-tested, and used under different programs in developing countries. The brief review of these technologies is intended as an update of the technological developments in arsenic testing, arsenic removal, and alternative water supplies. It is hoped that the review will be of assistance to those involved in arsenic mitigation in South and East Asian countries.
CAUSES AND INCIDENCES
Arsenic is a chemical metallic element with symbol As an atomic number 33. It forms a number of poisonous compounds and is found in nature at low levels mostly in compounds with oxygen, chlorine, and sulfur. These are called inorganic arsenic compounds. Arsenic is present in the environment and humans all over the world and is exposed to small amounts, mostly through food, water, and air. Contamination of groundwater, either from anthropogenic or natural sources with several social impacts, has now turned to be a major environmental concern in different parts of the world. Millions of people in several countries are exposed to high levels of As through intake of As-rich groundwater. Elevated level of As in groundwater has been well documented in Chile, Mexico, China, Argentina, USA, and Hungary[1,2] as well as in the Indian State of West Bengal, Bangladesh, and Vietnam.[2-6] About 150 million people around the world are estimated to be affected globally with an increasing prospect as new affected areas are continuously discovered.[7] Groundwater contamination is almost continuously the aftereffect of human activities. In zones, where population density is high and human utilization of the land is concentrated, ground water is particularly vulnerable. Virtually any activity whereby chemicals or wastes may be released to the environment, either intentionally or accidentally, has the potential to pollute groundwater (EPA/625/R-93/002). At the point, when groundwater becomes to be distinctly polluted, it is difficult, especially for the rural households, supplied with scattered hand pump tube wells. Thereafter, it has become a major public health problem throughout Southeast Asia, particularly more severe at the high-dense population areas around the greater Dhaka, Chittagong, and Khulna regions of Bangladesh, and therefore, is a great burden on water supply authorities. In developing countries like India and Bangladesh, the high prevalence of contamination, the isolation and poverty of the rural population, and the high cost and complexity of arsenic removal systems have imposed a programmatic and policy challenge on an unprecedented scale.[8,9]
Recent studies in Bangladesh indicate that the groundwater is severely contaminated with arsenic above the maximum permissible limit of drinking water. In 1996, altogether 400 measurements were conducted in Bangladesh.[10] Arsenic concentrations in about half of the measurements were above the maximum permissible level of 0.05 mg/l in Bangladesh. In 1998, the British Geological Survey (BGS) collected 2022 water samples from 41 arsenic-affected areas including the major districts of over-populated Dhaka and Chittagong divisions.[10,11] Laboratory tests revealed that 35% of these water samples were found to have arsenic concentrations above the level of 0.05 mg/l.[11]
PUBLIC HEALTH THREATS
Arsenic contamination in the environment is turning to be a serious public health and social problem in several parts of the world. It is well-established fact that arsenite AsIII is more toxic than arsenate AsV, with inorganic As being more toxic than organic As.[12] However, different organic As species represent different degrees of toxicity.
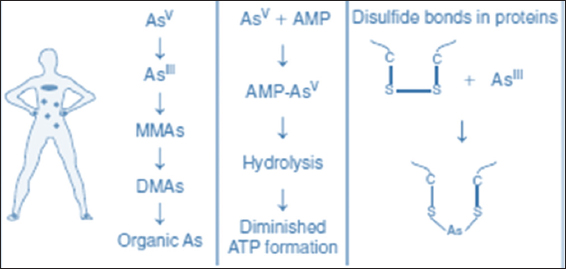
According to a survey performed by the BGS and the Department of Public Health Engineering (DPHE), over 92 million people in Bangladesh alone are exposed to drinking water with elevated As concentrations.[13] The problems caused by As in the groundwater of Southeast Asian countries are far-reaching and multifaceted. The most widely known effect is health issues [Figure 1].
Figure 1: Mechanisms of As toxicity inside of the body
A common name generally used for arsenic-related health problems includesskin disorders, skin cancers, internal cancers (bladder, kidney, and lung), diseases of the blood vessels of the legs and feet, possibly diabetes, increased blood pressure, and reproductive disorders.[14-16] Arsenic exerts detrimental effects on general protein metabolism with high toxicity by reacting with sulfhydryl groups existing in the cysteine residues.[17] Arsenicosis causes also dire consequences for the livelihood, family life, and earning capability when individuals fall victim. The absence of taste, odor, color, and exposure makes arsenic impossible for a layman to detect and avoid. Applying the WHO provisional guideline for drinking water of 10–50 ppb of arsenic, a population of more than 100 million people worldwide is at risk, and of these, more than 45 million people mainly in developing countries from Asia are at risk of being exposed to more than 50 ppb of arsenic, which is the maximum concentration limit in drinking water in most of the countries in Asia.[7]
SOCIOECONOMIC EFFECTS
The presence of As in irrigation and drinking water in South and Southeast Asia also causes severe socioeconomic problems.[18] At larger perspectives, elevated As contamination of a region may result in societal stress, disability in individuals, poverty, and decreased market value of potentially contaminated agricultural products leading to low income to the affected farmers. The social acceptance of people showing visual signs of arsenicosis often decreases[19] as non-infected villagers unknowingly suspect leprosy and avoid the person.[20] Deterioration in physical appearance also makes women socially excluded. Life becomes miserable if one of the partners of a married couple is arsenic affected, he/she is often divorced, and sent back to their parents, sometimes together with their children.[20,21] Wedding arrangements are cancelled, or no matrimonial agreement can be made for affected sons and daughters.[21] People with obvious skin lesions are not offered jobs[20] or are asked to leave.[19] Often affected individuals only dare leaving the house at night. In particular, people with a lower income and subsequently higher dietary deficiencies are more often struck by arsenicosis,[22] decreasing the chances of a better life for these people even more.[19] The production of food in terms of yield and quality is also notably impaired in South and Southeast Asia.[23,24] In Bangladesh, 4 million hectares of the agriculturally used land is under irrigation, of which 3 million hectares are watered with tube well water.[23] Bangladeshi soils contain background contents of 4–8 mg As per kilogram, although these levels can increase substantially in areas, where As-contaminated water is used for irrigation.[25-28] Agricultural products, especially the mainly grown crop rice, accumulate a lot of As in the edible parts.[29] Constant As exposure to rice plants results in a decreased growth yield, grain number, and size.[25,30-33] In fact, the uptake of too much As by rice would completely inhibit grain formation, which is known as straight head disease.[34] Khan et al.[33] have shown that irrigating paddy soils with As-containing irrigation water leads to a higher bioavailability of As in these soils, and hence, to a lower biomass of the rice. A loss in rice yield would negatively affect agricultural sustainability, national economies, and the food security and nutritional status of the farmers,[24,25,33-35] as rice accounts for 76% of their average calorie intake.[36] However, as Asian rice is exported worldwide, the health of people from other parts of the world could also be negatively affected.[35]
MITIGATION TECHNOLOGIES
Selection of appropriate method to supply water with reduced As content relies on several factors and is complicated as the majority of the affected population lives in rural areas is deprived off infrastructure and with decentralized water supplies from millions of shallow tube wells extracting water from shallow aquifers. Mitigation strategies for As contamination problem in groundwater, therefore, should address both technological and the socioeconomic considerations.[37]
However, the basic principles of arsenic removal from water are based on conventional techniques of oxidation, co-precipitation and adsorption on coagulated flocs, adsorption onto sorptive media, ion exchange, and membrane filtration. Oxidation of As(III) to As(V) is needed for effective removal of arsenic from groundwater by most treatment methods. The most common arsenic removal technologies in Bangladesh (including other Southeast Asian countries) can be grouped into the following categories:
-
Oxidation and sedimentation
-
Coagulation and Filtration
-
Sorptive filtration.
Oxidation and Sedimentation
Most treatment methods are effective in removing arsenic in pentavalent form, and hence, include an oxidation step as pretreatment to convert arsenite to arsenate. Arsenite can be oxidized by oxygen, ozone, free chlorine, hypochlorite, permanganate, hydrogen peroxide, and Fulton’s reagent, but atmospheric oxygen, hypochloride, and permanganate are commonly used for oxidation in developing countries. The oxidation processes convert predominantly non-charged arsenite to charged arsenate, which can be easily removed from the water. Atmospheric oxygen is the most readily available oxidizing agent, and many treatment presses prefer oxidation by air. However, air oxidation of arsenic is a very slow process and it can take weeks for oxidation to occur.[38] Air oxidation of arsenite can be catalyzed by bacteria, strong acidic or alkali solutions, copper, powdered activated carbon, and high temperature.[39]
Chemicals such as chlorine and permanganate can rapidly oxidize arsenite to arsenate under a wide range of conditions. Hypochloride is readily available in rural areas, but the potency (available chlorine) of the hypochloride decreases when it is poorly stored. Potassium permanganate is also readily available in developing countries. It is more stable than bleaching powder and has a long shelf life. Ozone and hydrogen peroxide are very effective oxidants, but their use in developing countries is limited. Filtration of water through a bed containing solid manganese oxides can rapidly oxidize arsenic without releasing excessive manganese into the filtered water.
In situ oxidation of arsenic and iron in the aquifer has been tried in Bangladesh under the Arsenic Mitigation Pilot Project of the DPHE and the Danish Agency for International Development (Danida). The aerated tube well water is stored in feed water tanks and released back into the aquifers through the tube well by opening a valve in a pipe connecting the water tank to the tube well pipe under the pump head. Chlorine and potassium permanganate are used for oxidation of As(III) to As(V) in many treatment processes in Bangladesh and India. Solar oxidation and removal of arsenic is a simple method of solar oxidation of arsenic in transparent bottles to reduce arsenic content of drinking water.[40] Ultraviolet radiation can catalyze the process of oxidation of arsenite in the presence of other oxidants such as oxygen.[41] Experiments in Bangladesh show that the process on average can reduce the arsenic content of water to about one-third of the original concentration.[8] Arsenic reduction by plain sedimentation appears to be dependent on water quality, and in particular, the presence of alkalinity and precipitating iron in water. Passive sedimentation, in most cases, failed to reduce arsenic to the desired level of 50 µg/L in a rapid assessment of technologies conducted in Bangladesh.[42]
Coagulation and Filtration
In the process of coagulation and flocculation, arsenic is removed from solution through three mechanisms:
-
Precipitation: The formation of insoluble compounds.
-
Coprecipitation: The incorporation of soluble arsenic species into a growing metal hydroxide phase.
-
Adsorption: The electrostatic binding of soluble arsenic to external surfaces of the insoluble metal hydroxide.[39]
Precipitation, coprecipitation, and adsorption by coagulation with metal salts and lime followed by filtration are a well-documented method of arsenic removal from water. This method can effectively remove arsenic and many other suspended and dissolved solids from water, including iron, manganese, phosphate, fluoride, and microorganisms, reducing turbidity, color, and odor and resulting in a significant improvement in water quality. Thus, the removal of arsenic from water using this method is associated with other ancillary health and esthetic benefits. Water treatment with coagulants such as aluminum alum (Al2[SO4]3.18H2O), ferric chloride (FeCl3), and ferric sulfate (Fe2 [SO4]3.7H2O) is effective in removing arsenic from water. Oxidation of As(III) to As(V) is required as a pre-treatment for efficient removal. It has been suggested that preformed hydroxides of iron and aluminum remove arsenic through adsorption, while in situ formation leads to coprecipitation as well.[39] In alum coagulation, the removal is most effective in the pH range of 7.2–7.5, and in iron coagulation, efficient removal is achieved in a wider pH range, usually between 6.0 and 8.5.[43] The effects of cations and anions are very important in arsenic removal by coagulation. Anions compete with arsenic for sorptive sites and lower the removal rates. Manning and Goldberg[44] indicated the theoretical affinity at neutral pH for anion sorption on metal oxides as: PO4 > SeO3 > AsO4 > AsO3 >> SiO4 > SO2 > F > B(OH)3. The presence of more than one anion can have a synergistic effect on arsenic removal. Addition of either silicate or phosphate has some effects on arsenic removal, but the presence of both can reduce arsenate removal by 39% and arsenite removal by 69%.[45] Based on arsenic removal studies in Bangladesh, Meng and Korfiatis[46] concluded that elevated levels of phosphate and silicate in Bangladesh well water dramatically decreased adsorption of arsenic by ferric hydroxides.
The technologies developed based on the coagulation-sedimentation-filtration process include:
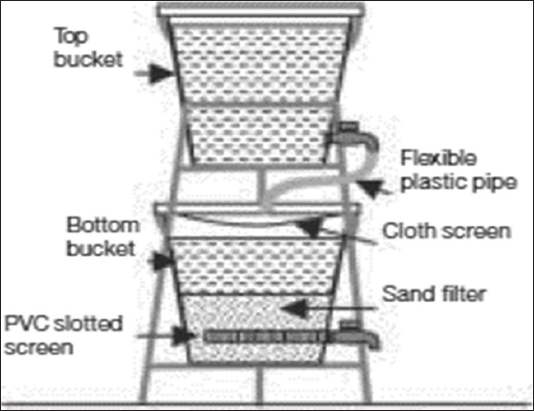
The bucket treatment unit, developed by the DPHE-DANIDA project and improved by the Bangladesh University of Engineering and Technology (BUET), is based on coagulation, coprecipitation, and adsorption processes.
Sorptive Filtration
Several sorptive media have been reported to remove arsenic from water. As with the coagulation process, pre-chlorination improves the column capacity dramatically.
The activated alumina-based sorptive media used in Bangladesh and India include:
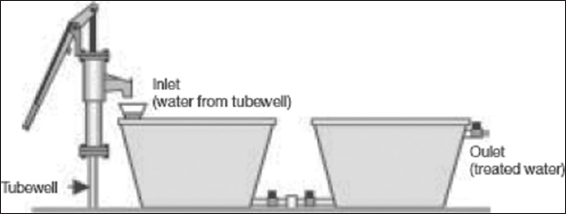
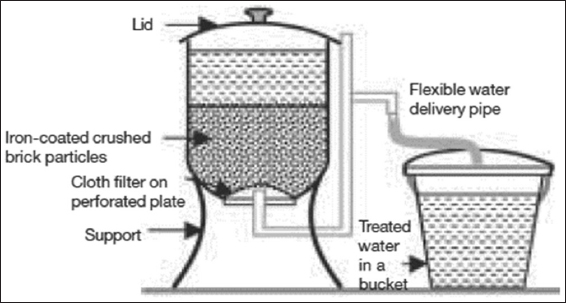
The BUET iron-coated sand filter was constructed and tested on an experimental basis and found to be very effective in removing arsenic from groundwater. The unit needs pre-treatment for the removal of excess iron to avoid clogging of the active filter bed. Iron-coated sand is prepared following a procedure similar to that adopted.[53] The Shapla arsenic filter [Figure 5], a household-level arsenic removal unit, has been developed and is being promoted by the International Development Enterprises, Bangladesh.[48]
Figure 5: Shapla filter
The READ-F arsenic filter is promoted by Shin Nihon Salt Co. Ltd., Japan, and Brota International Services, Bangladesh, for arsenic removal in Bangladesh. READ-F displays high selectivity for arsenic ions under a broad range of conditions and effectively adsorbs both arsenite and arsenate. The READ-F is ethylene-vinyl alcohol copolymer-borne hydrous cerium oxide in which hydrous cerium oxide (CeO2.nH2O) is the adsorbent. Laboratory tests at the BUET and field testing of the materials at several sites under the supervision of the BAMWSP showed that the adsorbent is highly efficient in removing arsenic from groundwater.[54] One household treatment unit and one community treatment unit based on the READ-F adsorbent are being promoted in Bangladesh.[48] The units need iron removal by sand filtration to avoid clogging of the resin bed by iron flocs. Hence, the development of ion-specific resin for exclusive removal of arsenic can make the process very attractive. Furthermore, the well-known American company (Tetrahedron, Inc.) promoted ion exchange-based arsenic removal technology, which has been reported to be partly used in Bangladesh.[48]
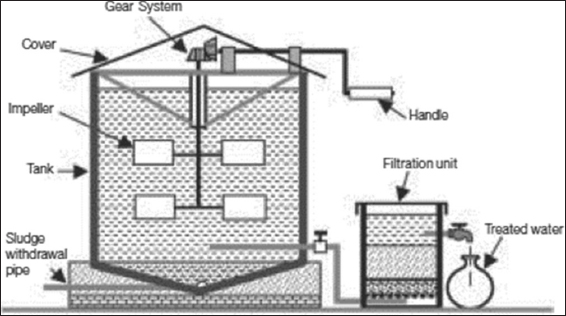
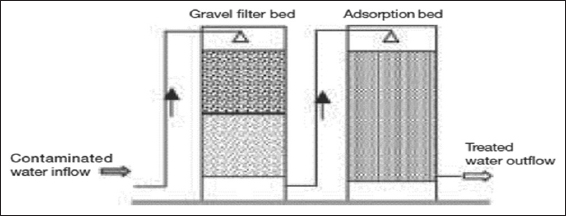
Granular ferric hydroxide (AdsorpAs) is a highly effective adsorbent used for the adsorptive removal of arsenate, arsenite, and phosphate from natural water. It has an adsorption capacity of 45 g/kg for arsenic and 16 g/kg for phosphorus on a dry weight basis.[52] M/S Pal Trockner (P) Ltd., India, and Sidko Limited, Bangladesh, have installed several granular ferric hydroxide-based arsenic removal units in India and Bangladesh. The typical arrangement of the Sidko/Pal Trockner unit [Figure 6] requires aeration for oxidation of water and pre-filtration for the removal of iron flocs before filtration through active media. Chemicon and Associates have developed and marketed an arsenic removal plant based on adsorption technology in which crystalline ferric oxide is used as an adsorbent. The unit has a pre-filtration unit containing manganese oxide for oxidation of As(III) to As(V) and retention of iron precipitates.
Figure 6: Granular ferric hydroxide-based arsenic removal unit
CUMULATIVE COSTS
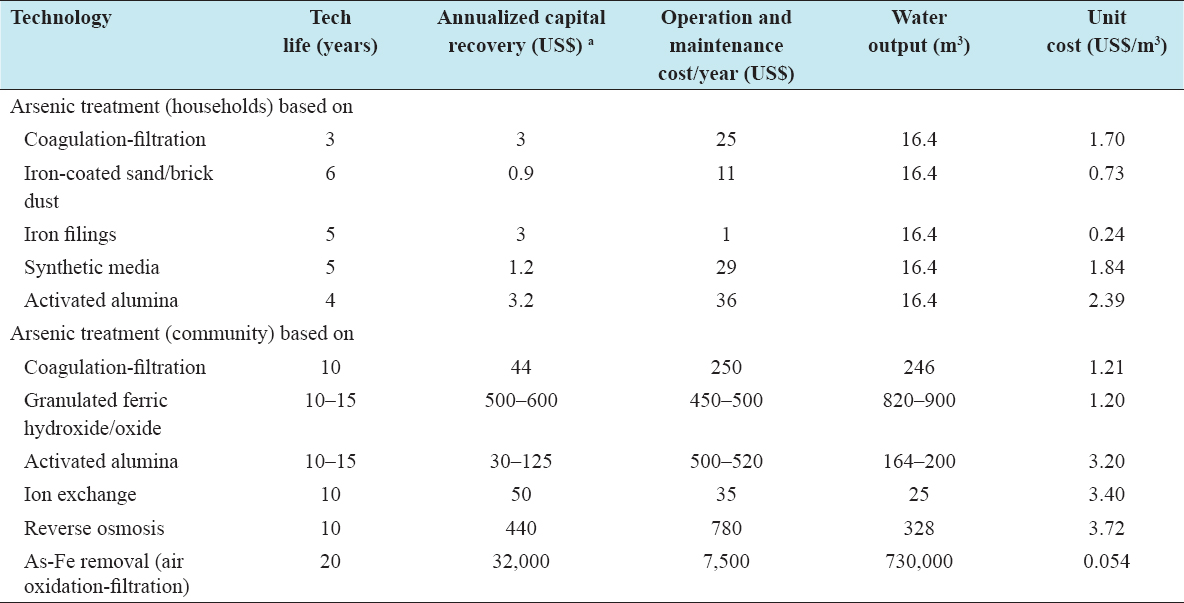
The cost of arsenic removal technology is an important factor for its adoption and sustainable use in rural areas. The cost of the technologies depends on many factors such as the materials used for fabrication of components, quantity of media or chemicals used, and quality of groundwater. Most of the technologies have been installed and are being operated under field testing and pilot scale operations. A study in Bangladesh shows that a low-income household is willing to pay about US$ 0.75 per month for arsenic-safe water, which is not adequate even to meet the O and M costs of most water supply systems.[55] The cost of arsenic removal with iron by simple aeration-filtration is comparatively low, but the efficiency of the method is dependent on the presence of iron and optimum alkalinity in natural water. Hence, the costs of installation, operation, and maintenance of all the arsenic removal systems are not known or are yet to be standardized based on modifications to suit the local conditions. The unit costs of water produced by different water supply systems to meet present service levels have been calculated on the basis of annualized capital recovery using an annual interest rate of 12% [Table 1]. It has been assumed that the arsenic-safe water required per family for drinking and cooking is 45 L/day. If the full water production capacities of these systems are utilized, the cost per unit volume of water is greatly reduced.
Table 1: Cost of water supply options for arsenic mitigation
CONCLUSION AND RECOMMENDATION
Arsenic contamination of groundwater is an alarming problem on a global scale. In several parts of the world, biogeochemical processes have resulted in the dissolution of naturally occurring As into groundwater. To combat the problem, various remediation methods and technologies for removal of As in several areas of the Southeast Asian countries have been critically reviewed. Most of the existing technologies for the removal of As involve the direct removal of AsV or converting AsIII to AsV followed by removal of AsV. The implementation of mitigation options can be facilitated by setting proper guidelines and to control implementation at appropriate intervals. Modifications based on pilot-scale implementation of the technologies are in progress with the following objectives:
-
Improving efficiency of arsenic removal.
-
Reducing capital and operation cost of the systems.
-
Making the technology user-friendly.
-
Overcoming maintenance problems.
-
Resolving sludge and arsenic concentrates management problems.
The technologies are site specific, and there are various considerations for selection of a particular technology in any given locality. Some of the important considerations for the development of sustainable water supply options for purposes of arsenic mitigation are as follows:
-
The profile of the beneficiaries and settlement pattern.
-
Present water supply system and level of arsenic in the drinking water.
-
Possible alternative sources of water for water supply.
-
Relative risk and cost of development of water supply system.
-
The level of technical and managerial capacity building needed.
-
Affordability and willingness to pay.
Governmental and donor-based financial and logistic assistance may be essential to reduce arsenicosis. Besides, extensive research should address the understanding of the occurrence, origin, and distribution pattern of arsenic. We sincerely hope that this paper will be of considerable interest to the readers. The paper reflects the latest state of the art on understanding of various interdisciplinary facets of the problem of arsenic in environmental realm, mechanisms of mobilization in groundwater, biogeochemical interactions, and the measure for remediation.