INTRODUCTION
Depression is a common comprehensive mental disorder. The core symptoms of depression are characterized by weight loss, low morale, anhedonia, and disrupted sleep.[1] There are more than 300 million people living with depression now, an increase of more than 18% between 2005 and 2015.[2] The curative effects are far away from satisfactory, which primarily reflected in slow work (weeks to months), low efficiency (only one-third of patients achieve full remission of their depressive symptoms and gain functional recovery) and many side effects (insomnia, headache, and anxiety).[3] Therefore, it has become a research hotspot to explore other mechanisms of depression and develop new natural antidepressants with high efficiency and low toxicity.
In Malaysia, Ficus deltoidea (FD) is commonly known as Mas cotek. It is an epiphytic shrub which has been traditionally used to treat sores, wounds, pain, diabetes, disorders of the menstrual cycle, and rheumatism.[4] It was reported that FD possessed antioxidant activity in vitro models[5,6] and anti-inflammatory activity.[7,8] Oxidative stress and inflammation play a key role in the pathophysiology of depression.[9,10] We speculate FD extract possesses antidepressant-like effect due to its traditional efficacy and chemical composition. In this study, chronic unpredictable mild stress (CUMS)-treated rats were used to investigate the antidepressant-like effect of FD treatment.
MATERIALS AND METHODS
Animals
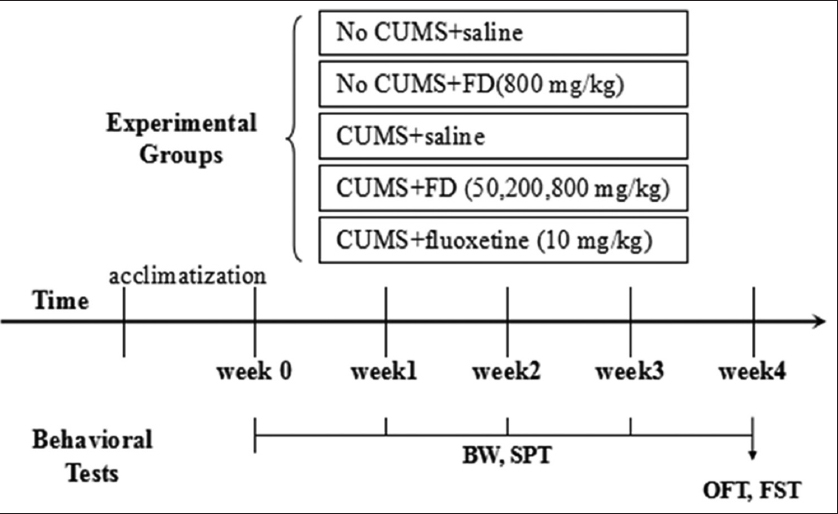
Forty-two healthy adult male Sprague-Dawley rats (200-220 g, 6-8 weeks) were supplied by TAKRIF BISTARI ENTERPRISE (Malaysia) and housed with 12 h illumination, 25 ± 1°C room temperature and free access to water and food. After 7-day acclimatization to their new environmental conditions, the rats were randomly divided into control, CUMS, FD alone (800 mg/kg), different doses (50, 200, and 800 mg/kg) of FD, and fluoxetine (10 mg/kg, positive control) groups. There were six rats in every group. There was no stress in control and FD alone group. The rats in other groups were subjected to the following stressors once a day for 28 days: Inversion of the light/dark cycle, wet bedding for 24 h, nip tail for 1 min, shaking for 10 min, water deprivation for 24 h, fasting for 24 h, swimming in 45°C hot water for 5 min, swimming in 4°C cold water for 5 min, and cage tilting for 24 h.[11,12] A stressor was performed randomly every day and the same stressor could not appear continuously. The animals were given oral administration once a day for 28 days. Rats in control and CUMS group were given normal saline with equal volume (Figure 1).
Figure 1: Schematic representation of the chronic unpredictable mild stress (CUMS) experimental procedure and behavioral tests. After 4 weeks of CUMS procedure or control condition, rats were performed behavioral tests before being sacrificed. The brains, kidneys, and livers were then collected for morphology
Drug Administration
The leaves of FD were collected from a farm in Malacca. After being air-dried, the leaves were coarsely powdered, and then, extracted with boiling water for 1 h. The infusion was filtered, and the filtrate was spray-dried to form a powder. FD and fluoxetine (Zhongxi Pharmaceutical Co., Ltd. Shanghai, China) was diluted in sterile water to suitable concentration. All treatments were administered 30 min before stress exposure.
Detection of Body Weight
Body weight of rats in every group was monitored on 0 day, 7 days, 14 days, 21 days, and 28 days of the experiment.
Sucrose Preference Test (SPT)
All rats were applied adaptation to sucrose solution 2 days before SPT. Two bottles were put in every cage at the same time. One was 1% sucrose solution, the other was water. To avoid the preference of the position where the rats drank, the position of the two bottles was exchanged every 12 h. After rats were deprived of water for 24 h, the volumes of consumed water and sucrose solution were recorded in 2 h. The sucrose preference (%) = sucrose consumption/(water consumption + sucrose consumption).
Open Field Test (OFT)
After 1 h of habituation to the test room, the rats were placed in a black square open box (100 × 100 × 40 cm) one by one, of which the base consisted of 25 same squares. When the rat was put at the center of the box, the locomotor activities (defined as locomotion on at least three paws) and rearing activities (defined as an upright posture sustained on hind paws) of the rat in 5 min were recorded with a video-computerized tracking system.
Forced Swimming Test (FST)
Rats were subjected to an open cylindrical container (height 45 cm, diameter 18 cm) filled with water (depth 35 cm) at 25 ± 2°C. The activities of rats in 6 min were recorded with a video-computerized tracking system. After each test, rats were dried by towels and returned into their cage under a heater. The immobility time was defined when no obvious activity was observed except essential activity to keep the head out of water.
Morphology of Hippocampus, Liver, and Kidney with hematoxylin-eosin (HE) Staining
After behavioral tests, 10% chloral hydrate (0.3 mL/100 g, i.p.) was used to anesthetize the animals. Hippocampus was rapidly separated from the skull. Kidneys and livers were also removed from peritoneal cavity. Then, the tissues were fixed in 4% paraformaldehyde, manufactured into paraffin specimen, cut into 6 μm thick sections, and stained with HE. Light microscopy (Olympus, U-25ND6) was used to observe the morphology of liver, kidney, and hippocampal neurons in CA1 region in each group. Pyknotic hippocampal neurons (%) = the number of pyknotic neurons/the number of total neurons in the same field of microscope.
Statistical Analysis
Data were presented as mean ± standard deviation and analyzed with the GraphPad Prism 5 software. One-way ANOVA followed by the Tukey post-hoc test was used for statistical analysis of the results as appropriate. Significance level was set at P < 0.05.
RESULTS
The Effects of FD on Body Weight of CUMS-treated Rats
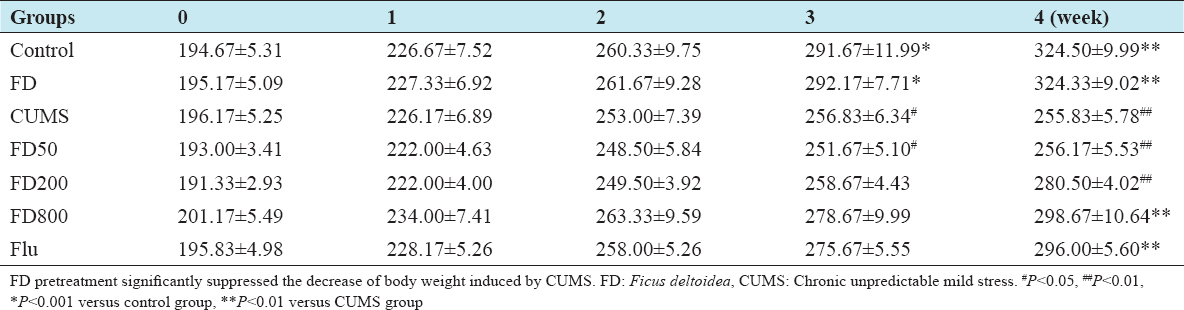
As shown in Table 1, there was no significant difference in each group for body weight at the beginning of the experiment (P > 0.05). Compared with the control group, CUMS treatment resulted in a significant reduction in body weight increase from the 3rd week (P < 0.05). At the 4th week, the average body weight of rats in CUMS group was reduced by 21.30%, being 255.83 ± 5.78 g for the CUMS group and 324.50 ± 9.99 g for the control group. The decrease of body weight induced by CUMS was ameliorated by the pretreatment of FD (800 mg/kg) or fluoxetine (10 mg/kg) (P < 0.01).
Table 1: Effect of FD on body weight of CUMS-treated rats (g) (n=6)
The Effects of FD on SPT of CUMS-treated Rats
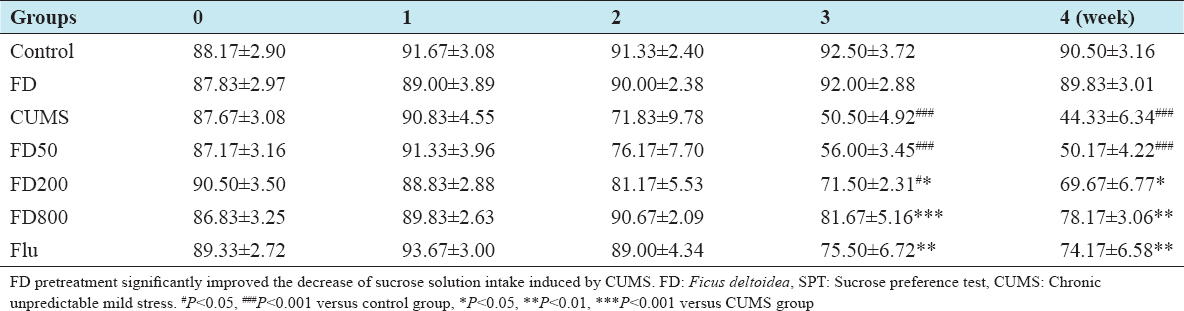
There was no significant difference in each group for sucrose solution intake at the beginning of the experiment (P > 0.05). The sucrose preference of rats in CUMS group tended to decrease over time and was notably reduced compared with that of scheme group from the 3rd week (P < 0.001). This indicated anhedonia and decreased response capability to happiness events. The sucrose solution intake of rats in FD200 (P < 0.05), FD800 (P < 0.01), and flu (P < 0.01) group increased considerably at the 4th week compared with CUMS group (Table 2).
Table 2: Effect of FD on SPT of CUMS-treated rats (%) (n=6)
The Effects of FD on OFT of CUMS-treated Rats
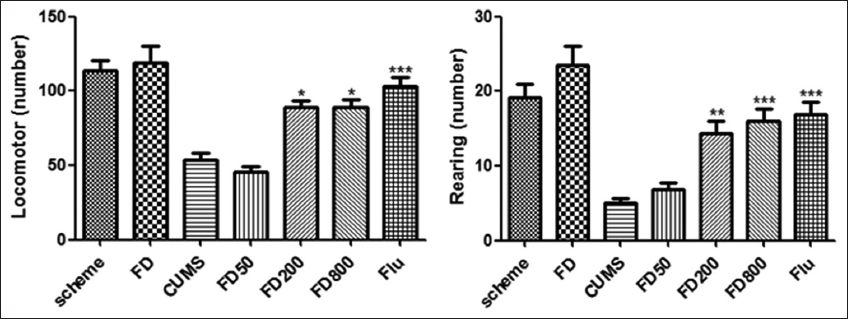
Compared with the control group, the locomotor and rearing activities number of rats in CUMS group decreased significantly (P < 0.01). Decreased locomotor and rearing activities indicated that animals showed depression, loss of interest, and lack of pleasure. The locomotor and rearing activities number of rats in FD (200, 800 mg/kg) and flu group were significantly increased (P < 0.01, P < 0.001, respectively) compared with CUMS group (Figure 2).
Figure 2: Effect of FD on OFT of CUMS-treated rats. FD pretreatment significantly increased the number of locomotors and rearing activities compared with CUMS group. FD: Ficus deltoidea, OFT: Open field test, CUMS: Chronic unpredictable mild stress. **P < 0.01, ***P < 0.001 versus CUMS group
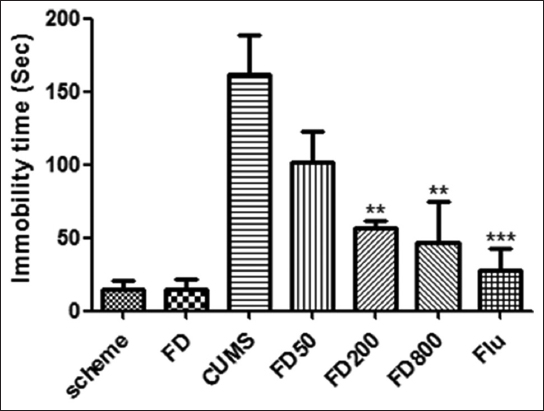
The Effects of FD on FST of CUMS-treated Rats
Compared with the control group, the immobility time of rats in CUMS groups was significantly longer during the 6-min test period (P < 0.001). The rats in FD (200, 800 mg/kg) or flu (10 mg/kg) group spent less time immobile compared with CUMS group (P < 0.01, P < 0.001, respectively) (Figure 3).
Figure 3: Effect of FD on FST of CUMS-treated rats. A significant decrease in floating behavior was observed in rats pretreated with FD (200, 800 mg/kg) and flu compared with CUMS group. FD: Ficus deltoidea, FST: Forced swimming test, CUMS: Chronic unpredictable mild stress. **P < 0.01, ***P < 0.001 versus CUMS group
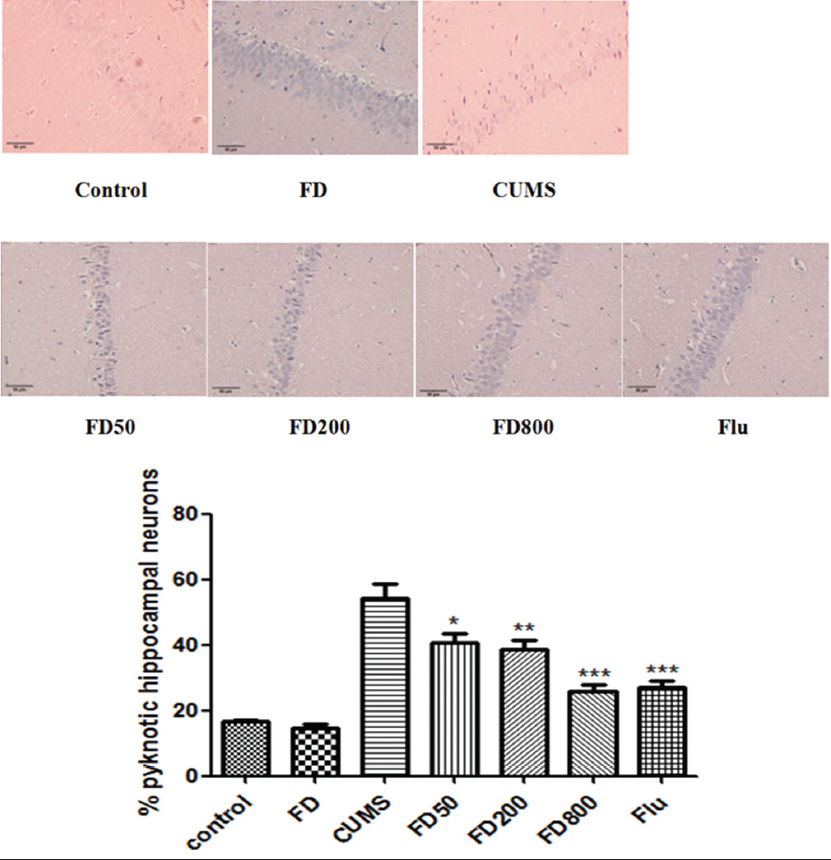
Neuronal Morphology in the CA1 Region of Hippocampus
Neurons in control group were normal, with a clearly round appearance, intact membranes, a clear cytoplasm, a clear nucleus, and distinct nucleoli. Compared with the control group, the density of dark stained and pyknotic hippocampal neurons in CA1 region was significantly increased in CUMS group. In hippocampal CA1 region of rats in CUMS group, we also observed nucleus shrinkage or disappearance and neuron loss. However, the amount of pyknotic cells was significantly decreased in FD group, especially 800 mg/mL, compared with CUMS group (Figure 4).
Figure 4: Effects of FD on neuronal cell survival in CA1 region in hippocampus of CUMS-treated rats. FD pretreatment significantly decreased the number of dark stained and pyknotic hippocampal neurons in CA1 region compared with CUMS group. Representative photographs of CA1 region of hippocampus stained by HE in each group (original magnification ×200). The bar graphs reflected the rate of pyknotic and dark-stained neurons in CA1 region in each group. Data were presented as mean ± SEM. FD: Ficus deltoidea, CUMS: Chronic unpredictable mild stress, SEM: Standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001 versus CUMS group
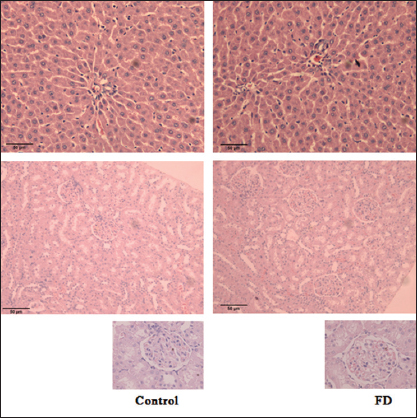
Morphology of Liver and Kidney
As shown in Figure 5, glomerular with normal volume formed regularly. Renal corpuscles were clear. There was no abnormality of the number of mesangial cells, endothelial cells, and epithelial cells. The apical end of each proximal tubule cell has a brush border of microvilli. The boundaries between adjacent proximal tubule cells are inconspicuous. Most of round nucleus located at the base. There was a usual appearance of a larger, clearer lumen in each distal tubule which had a lesser degree of acidophilia. The central vein located at the center of the lobule. Portal triads situated at the periphery of the lobule, which were composed of one or more small branches of the portal vein, a branch of the hepatic artery, and a small bile duct. Hepatocytes usually had one, sometimes two, large round to slightly oval nuclei, which were distinctly round, with one or two prominent nucleoli. There was no obvious difference of renal and liver histology between control and FD group.
Figure 5: Effects of FD on histology of liver and kidney. FD pretreatment had no obvious toxicity to liver and kidney. Representative photographs of liver and kidney stained by HE in control and FD group (original magnification ×100 and ×200). G: Glomerular, PT: Portal triads, FD: Ficus deltoidea, HE: Hematoxylin-eosin
DISCUSSION
Animal model of depression are often used to study the pathophysiology and symptomatology of depression, screen novel antidepressants, and identify the mechanism of antidepressants.[13] CUMS is one of widely used rodent depressed models because the behaviors of CUMS-treated animals approximate the symptoms of patients with depression such as appetite loss, weight changes, decreased exploratory activities, diminished cognitive functioning, anhedonia, and hopelessness.[11,12]
Rodents prefer sweet solutions or foods from birth. SPT can be used to evaluate the motivation and affective state of subject rats. Decreased preference for sweet solution in SPT means anhedonia.[14] OFT is an experiment used to assay anxiety-related and exploratory behavior of rodents.[15] During the test, rats were put in a bright and novel environment. The parameters, such as motion distance and the number of rearing, are often used to evaluate anxiety of the animals. FST is an experiment widely used to measure susceptibility to negative mood in depression research.[16] At first, rats kept on swimming and leaping to escape from the water-filled container. When they gave up, they developed an immobile posture. Immobility is thought as depression-like behavior or negative stress-coping strategy. In the present study, we adopted CUMS depression model to observe the effects of FD extract on depression-like behaviors of rats by detecting body weight, SPT, OFT, and FST. The results showed that rats exhibited many depressive-like symptoms after being exposed to CUMS for 4 weeks. The treatment of FD (200, 800 mg/kg) extract attenuated or reversed these changes including decreased body weight, sucrose intake and autonomous activities, and increased immobility time. These results suggested the rat model of depression was successful and FD extract possessed an anti-depressive effect on CUMS-treated rats.
It has been reported that CUMS treatment could induce neuronal degeneration in hippocampus which plays a key role in many respects of depression.[17,18] The present study exhibited similar findings after exposure to CUMS for 4 weeks. Treatment with FD (200, 800 mg/mL) decreased the number of dark stained and pyknotic hippocampal neurons in CA1 region, suggesting its potential neuroprotective effect against CUMS exposure.
Increased inflammation and oxidative stress are involved in the pathogenesis of depression[9] including the metabolism of neurotransmitters,[19-21] neuroendocrine function,[22] and synaptic plasticity.[17] Phytochemical studies confirmed FD contained numerous constituents’ classes, such as phenol,[23] terpenes, steroids, tannins, saponins, and flavonoids.[24] It has been reported that several tannins and flavonoids isolated from FD had been identified for their strong anti-inflammatory activity[25] and anti-oxidant activity.[23] The aqueous extract of FD leaves possess anti-inflammatory effects against various inflammatory responses.[25] Abdullah et al.[5] used three in vitro assays to examine the anti-inflammatory activity of standard extracts of different varieties of FD. Therefore, it is possible that the antidepressant-like effect of FD observed in our study may be due to its phenolics and flavonoids components. Moreover, the preliminary acute oral toxicity test showed that there is no observed adverse effect even at the dose of FD 2500 mg/kg.[26,27] This indicates that FD may have a considerable safety range to acute toxicity. In our study, different doses of FD showed no observable damage to kidney and liver. It is beneficial to the wide application of this plant in various disease conditions.
CONCLUSIONS
The present study indicated that CUMS caused depression-like behaviors of rats and increased neuronal degeneration in hippocampus. However, the aqueous extract of FD attenuates or reverses these changes. The results suggest the active compounds in FD and the mechanisms of its anti-depressant effects may be worth further investigating and elucidating.