INTRODUCTION
Epidemiological studies have diagnosed the incidence of colorectal cancer cases to about 40% annually and it is the third most leading cause of deaths among cancer patients worldwide.[1] Incidence of colorectal cancer is often associated with imbalance diet, lack of physical exercise, and high-fat consumption.[2] In addition, environmental chemical carcinogens induce free radicals, which alter the cell membrane potential, and produce an oxidative stress-related malignancy.[3] In response to the intra- and extra-cellular environmental conditions signaling pathways on the cell surface macromolecules are distorted.[4] Signaling molecules such as cell surface glycans are involved in many physiologically important functions that include normal embryonic development, differentiation, growth, contact inhibition, cell–cell recognition, host–pathogen interaction during infection, host immune response, disease development, metastasis, intracellular trafficking and localization, rate of degradation, and membrane rigidity.[5,6] Alterations on the surface glycans of the malignant cells undergo transformation to from glycolconjugates with cancer cells resulting in excessive replication potential. Energy created by this configuration is released through reactions with adjacent molecules, such as inorganic or organic chemical – proteins, lipids, carbohydrates – particularly with key molecules in membranes and nucleic acids.[7] Experimental colon cancer induced with 1, 2-dimethylhydrazine (DMH) produce free radical-mediated cancer cell glycolconjugates with increased byproducts leading to non-specific markers in cancer conditions.[8] A correlation between increased oxidative stress and improved glucose homeostasis states the combat of self-antioxidant defense system to eliminate the free radicals.[9] It is also well established that free radicals are known to react with all components of deoxyribonucleic acid (DNA), thus damaging its bases and the deoxyribose backbone causing mutations in crucial genes, which ultimately may lead to cancer.[10] Elevated levels of glycoconjugates express a direct relationship between glycoproteins and tumorogenesis.[11] Thereby, the excess glycoprotein level triggers the production of primary lysosomal enzymes beyond the control leading to degradation of the cells and ultimately causes cancer.[12] Studies have focused on the marker lysosomal enzymes, acid phosphatases, and many cathepsins, DNAases, ribonucleases, sulfatases, and glucuronidases are other important cellular lysosomal enzymes. Oxidative stress-related biochemical reactions have been implicated in cancer pathology, especially in colon cancer conditions were diet is predominantly considered to inhibit lysosomal enzymes.[13] Animal experimental studies have implicated that a large number of dietary phytochemicals may have anti-carcinogenic properties such as anti-oxidative action, induction of detoxification enzymes, and signal transduction.[14] Many dietary anti-carcinogens exhibit anti-oxidant properties. The compounds function by directly scavenging free radicals, altering the activity of antioxidant defense enzymes, or by affecting other biochemical processes involved in redox homeostasis. Of which monoterpenes are dietary components found in the essential oils of citrus fruits and other plants. A number of these dietary monoterpenes have antitumor activity. For example, d-limonene, which comprises >90% of orange peel oil, has chemopreventive activity against rodent mammary, skin, liver, lung, and fore-stomach cancers. Similarly, other dietary monoterpenes have chemopreventive activity against rat mammary, lung, and fore-stomach cancers when fed during the initiation phase.[15] The monoterpenes have several cellular and molecular activities that could potentially underlie their positive therapeutic index. Myrtenal a compound of monoterpenes was shown to have excellent pharmacological activities against many diseases among which cancer is imperative. Even though, it has been reported to have various biological activities, there is still a paucity of information, especially against the experimental colon cancer. Therefore, in light of the aforementioned reasons, this investigation was undertaken to evaluate the chemotherapeutic potential of myrtenal against DMH-induced colon carcinogenesis in Wistar Albino rats with reference to enzymes of carbohydrate metabolism, lysosomal enzymes, and nucleic acids.
MATERIALS AND METHODS
Reagents
DMH, myrtenal was purchased from Sigma Chemical Company, St. Louis, MO, USA. All the other chemicals used in this study were of analytical grade available commercially.
Experimental Animals
Experiments were carried out with 5-week-old male Wistar rats procured from central animal house facility, Dr. A. L. M. Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai - 600 113. They were maintained in the controlled environmental conditions of temperature and humidity on alternative 12 h light/dark cycle, noise level maintained below 85 db and had unrestricted access to standard diet consisting of 24% protein, 4.5% fat and 4% fiber. The experiment was sanctioned and approved by the Institutional Animal Ethical Committee (IAEC No. 01/13/2013).
Experimental Design
The experimental animals were divided into four groups, each group comprising six animals.
-
Group 1: Control animals fed with standard diet and pure drinking water
-
Group 2: Animals were administered with 20 mg/kg body weight (b.wt.) of DMH, in 1 mM ethylenediaminetetraacetic acid, pH ·adjusted to 6.5 with 1 mM NaOH and subcutaneously injected once in a week for 15 weeks
-
Group 3: Animals were treated with myrtenal (230 mg/kg b.wt.) with corn oil as vehicle for 30 weeks by intragastric administration. Myrtenal treatment was started 1 week before the first dose of 20 mg/kg b.wt. of DMH (as in Group 2)
-
Group 4: Animals were treated with myrtenal (230 mg/kg b.wt.) for 30 weeks by intragastric administration to assess the cytotoxicity if any, induced by myrtenal, and rats were referred as drug control.
After the end of the experimental period, the rats were fasted overnight and anesthetized using diethyl ether and sacrificed by cervical decapitation. A portion of colon was used for histopathological studies and remaining tissue was homogenized in 0.1 M Tris–HCl buffer pH 7.4 and centrifuged, the supernatant was used for biochemical studies.
Colon Analysis
Colon was excised from experimental rats, and were blotted dry and opened longitudinally, with the inner surface examined for visible macroscopic lesions. Tumor incidence (percentage of animals with tumors) and multiplicity (mean counted tumors per animals) were determined for the colons. Immediately following sacrifice, colons were removed and washed with ice-cold saline, and colon homogenates (10%) were prepared in ice-cold Tris-buffer saline (Tris 50 mM and NaCl 150 mM; pH 7.2) then centrifuged at 10,000 g for 10 min at 4°C and were stored as aliquots at or below −20°C for subsequent assays.
Biochemical Analysis
The glycoproteins such as hexose, hexosamines, and sialic acid were estimated according to the methods of Niebes[16] and Warren,[17] respectively. The absorbance was recorded at 540 nm and their levels were expressed as mg/dL for serum and mg/g for wet tissue. The nucleic acids were extracted by the method of Schneider[18] and the DNA was estimated by the method of Burton[19] and RNA was estimated by the method of Rawal et al.[20] The lysosomal enzymes such as the activities of β-D-galactosidase, β-D-glucuronidase, β-N-acetyl-D-glucosaminidase, and cathepsin-D were estimated according to the methods of Kawai and Anno,[21] Delvin and Gianetto,[22] Maruhn[23] and Sapolsky et al.,[24] respectively.
Histopathology - Periodic Acid and Schiff’s (PAS) Staining
Histochemical staining of glycoconjugates was carried out as per the method of Kierman (1990), using 2% PAS’s reagent in dark for 20 min. Photomicrographs were obtained using a Nikon Camera (Japan) to measure the relative intensity of PAS staining with the aid of a ×40 magnification lens in control and experimental groups.
Statistical Analysis
Values are expressed as mean ± standard deviation. The results were statistically evaluated using one-way analysis of variance by SPSS 10.0 student version followed by Tukey’s multiple comparison method to compare means of different groups. The mean difference is significant at the 0.05 levels.
RESULTS
Glycoprotein Contents in Colon Control and Experimental Animals
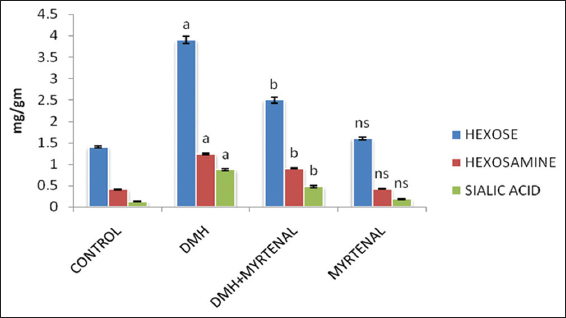
Effect of Myrtenal on the levels of hexose, hexosamines, and sialic acid in colon of control and cancer-bearing animals is shown in Figure 1. The levels of glycoproteins were significantly increased (P < 0.05) in Group II cancer-bearing animals, when compared with group I control animals. On the other hand, a considerable decrease in the glycoproteins levels were observed in Group III myrtenal treated animals when compared with Group II cancer-bearing animals. Whereas, no significant changes were noticed in Group IV myrtenal alone treated animals, when compared to Group I control animals.
Figure 1: Effect of myrtenal on levels of glycoproteins in colon of control and experimental animals. Values are expressed as mean ± standard deviation of six animals. Statistical significance P < 0.05. Control with 1, 2-dimethylhydrazine (DMH), DMH with DMH + myrtenal. Units: mg/g tissue
Nucleic Acid Content in Colon of Control and Experimental Animals
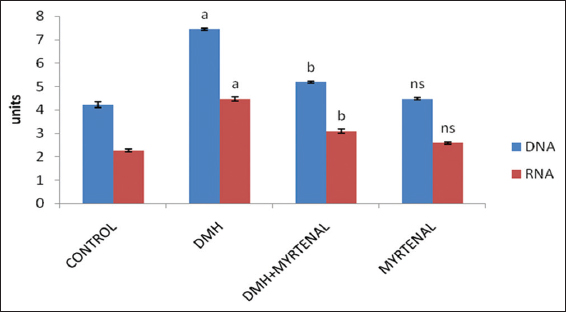
The effect of myrtenal on the levels of nucleic acids (DNA and RNA) in colon of control and experimental animals are shown in Figure 2. In Group II cancer-bearing animals, the levels of nucleic acids were found to be significantly elevated (P < 0.05) in colon tissues, when compared to Group I control animals. Conversely, these elevated levels were significantly decreased in myrtenal treated Group III animals (P < 0.05) when compared to Group II cancer-bearing animals. However, no significant changes were observed in Group IV myrtenal alone treated animals when compared to the Group I control animals.
Figure 2: Effect of myrtenal on the levels of nucleic acids in colon of control and experimental animals. Values are expressed as mean ± standard deviation of six animals. Statistical significance P < 0.05. Control with 1, 2-dimethylhydrazine (DMH), DMH with DMH + myrtenal. Units: mg/g wet tissue
Levels of Lysosomal Enzymes in Colon of Control and Experimental Animals
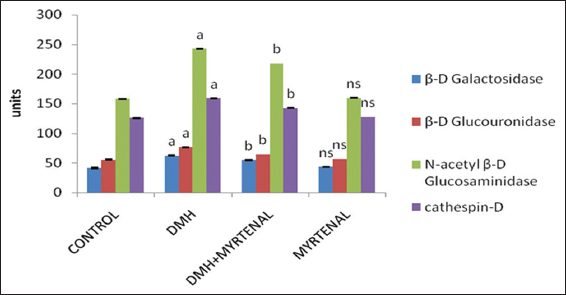
The effect of myrtenal on lysosomal enzyme activities in colon tissues of control and experimental animals are presented in the Figure 3. The activities of lysosomal enzymes were significantly increased in Group II cancer-bearing animals (P < 0.05) when compared to Group I control animals. On the contrary, the lysosomal enzyme levels were significantly decreased toward normal range (P < 0.05) in Group III myrtenal treated animals when compared with Group II cancer-bearing animals. Whereas, no significant alterations were observed in Group IV myrtenal alone treated animals when compared to Group I control animals.
Figure 3: Effect of myrtenal on the levels of lysosomal membrane in colon of control and experimental animals. Values are expressed as mean ± standard deviation of six animals. Statistical significance P < 0.05. Control with 1, 2-dimethylhydrazine (DMH), DMH with DMH + myrtenal. Units: β-D galactosidase (p-nitrophenol liberated/mg protein/h), β-D glucouronidase (p-nitrophenol liberated/mg protein/h), β-D glucosaminidase (p-nitrophenol liberated/mg protein/h), cathespin (p-nitrophenol n moles of tyrosine/mg protein/h)
PAS’s Staining Findings
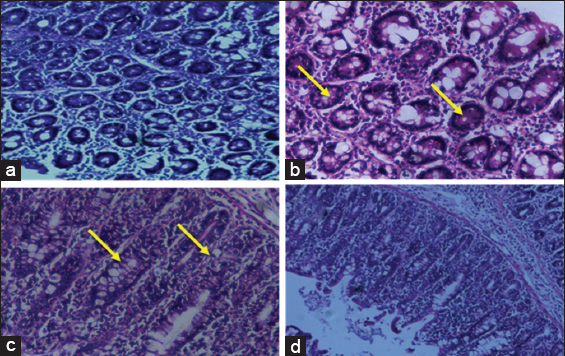
The effect of myrtenal in colon tissues of control and experimental animals are presented in the Figure 4. With effect of the PAS staining on the glycoprotein the intensity of mucin content are depicted. The normal architecture was seen profound in Figure 4a control animals. The neutral mucin intensity clearly expresses the increased levels of glycoproteins in Figure 4b DMH-induced Group II colon cancer animals compared to Group I control animals. Group III myrtenal treated animals shows active morphology in Figure 4c compared to Group II animals. Whereas, no significant alterations were observed in Group IV myrtenal alone treated animals in Figure 4d when compared to Group I control animals.
Figure 4: Histochemical analysis by periodic acid Schiff’s staining in control and experimental animals. (a) Control, (b) 1, 2-dimethylhydrazine (DMH) induced, (c) DMH + myrtenal, (d) myrtenal. Arrows depicts the expression of glycoconjugates
DISCUSSION
Glycoproteins are integral membrane proteins which play a major role in cell differentiation and absorption of macromolecules.[25] The increased levels of glycoproteins associated with cancer produces changes in macromolecular structure affecting cell–cell and cell–matrix interactions associated with decreased elasticity and increased fluid filtration.[26] In our study, the increased levels of hexose, hexosamines, and sialic acid in Group II animals are well in accordance with the excess free radical generated by DMH in the liver leading to oxidative stress-related inflammatory damage to cellular membranes.[27] Elevated levels of glycoproteins arise as a result of chemical glycosylation with hydroxyl groups by the free radicals on lipid membrane. The levels were seen decreased in myrtenal-treated animals providing effective free radical scavenging activity and thereby stabilizing the cell membrane integrity in glycosylated moieties.[28] Lysosomes are components of the endocytic pathway responsible for storage and processing of digestive enzymes for terminal degradation of cellular material delivered to autophagosomes during cell renewal and cell death.[29,30] Lysosomes play a critical role in protein, lipid, and carbohydrate catabolism; plasma membrane recycling; lipid and sterol trafficking; antigen processing; and autophagy. Disruption of lysosomal membrane integrity, and release of lysosomal enzymes into the cytosol, can have grave cytotoxic consequences resulting in apoptotic or necrotic death.[31,32] Increased levels of lysosomal enzymes in the cellular environment are as a result of free radical-mediated enzyme leakage of lysosomal sacs observed with the biomarkers such as β-D-galactosidase, β-D-glucuronidase, N-acetyl-B-glucosaminidase, and cathespin-D in colon cancer-induced animals.[33] Comparatively myrtenal supplementation reduced the lysosomal enzyme activity, which may be due to the enzyme stability of the lysosomal sacs with raise to membrane potency.
Cells become cancer cells because of DNA damage. In a normal cell, when DNA is damaged the cell either repairs the damage or dies. In cancer cells, the damaged DNA is not repaired but proceeds with abnormalities in their DNA content leading to malignancy.[34] Abnormal cells grow out of control as a result of DNA damage in the cellular environment delivering them as a potential biological target for initiation of many carcinogenesis thereby leading to sensitive indicators.[35] Altered physiological behavior triggers the risk of more exposure to chemical carcinogens and reduce the repair leading to DNA adduct formation.[36] These findings clearly suggest the uncontrolled accumulation of DNA damage and lack of repair mechanism could proceed to colon cancer as initiated by DMH. The chemical adduct between DMH-DNA thus indicate the important role of DNA in carcinogenesis.[37] RNA levels were also found to be increased in cancer condition as DNA and RNA are directly related. The abnormal increased level of DNA may lead to an increased transcription thereby increasing the RNA levels in tumor cells.[38] Thus, upon myrtenal treatment the levels were reverted back to near-normal which supports the anti-tumor activity by inhibiting the progression of tumor growth with tumor DNA content in malignant conditions.
Histochemical studies with PAS staining shows the neutral mucin content in colon cancer-induced animals stating the increased levels of mucin in the mucous epithelial cells of colon compared to myrtenal treated animals. In conclusion, our study revealed that monoterpenes and its derivatives mainly myrtenal have possessed significant anti-carcinogenic and anti-oxidative property against DMH-induced colon carcinogenesis in Wistar Albino rats.