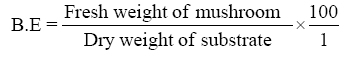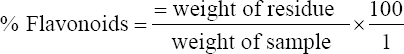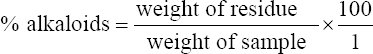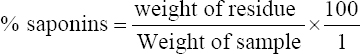1. Adesina FC, Fasidi IO, Adenipekum OC. Cultivation and fruit body production of Lentinus squarrosulus Mont (singer) on bark and leaves of trees, supplemented with agricultural waste. Afr J Biotechnol 2011;10:4608-11.
2. Jose N, Ajith TA, Janardhanan KK. Antioxidant, anti-inflammatory, and antitumor activities of culinary medicinal mushroom Pleurotus pulmonarius. Int J Med 2002;4:329-35.
3. Chang ST, Miles PG. Mushroom Cultivation, Nutritional Value, Medicinal Effect and Environmental Impact. 2nd ed. Boca Raton, Florida:CRC Press;2004. 451.
4. Zodrazil F, Burnnert F. Investigation of physical parameters important for the solid state fermentation of straw by white rot fungi. Eur J Appl Microbiol Biotechnol 1997;11:183-8.
5. Wood DA, Smith JF. The cultivation of mushroom. In:Norris JR, Pettipher GL, editors. Essays in Agricultural and Food Microbiology. Hoboken, New Jersey:John Wiley and Sons Ltd.;1997. 310-43.
6. Mane VP, Patil SS, Syed AA, Baig MM. Bioconversion of low quality lignocellulosic agricultural waste into edible protein by Pleurotus sajor-caju (Fr.) Singer. J Zhejiang Univ Sci 2007;8:745-51.
7. Rollon RJ, Batac RA, Batac RA, Maglines SM. Effects of carbonized rice hull and arbuscular mycorrhizal fungi application on potting media chemical properties, growth and nutrient uptake of Falcata (Paraserianthes falcataria L.). Int J Agron Agric Res 2018;13:93-101.
8. Lehmann J, Joseph S. Biochar for environmental management:An introduction. In:Biochar for Environmental Management. 2009. 1-12.
9. Baysal E, Baysal B, Pekerb H, Yalinkilica MK, Temizb A. Cultivation of oyster mushrooms on waste paper with some of added supplementary materials. Bioresour Technol 2003;89:95-7.
10. Iloka BC. Cultivation of Oyster Mushroom. Google Apps for Works;2013. 1-12.
11. Nwoko MC, Onyeizu UR, Okwulehie IC, Ukoima HN. Nutritional and bioactive compounds:Evaluation of Pleurotus pulmonarius (Fries) Quel. fruit bodies grown on different wood logs in Abia State, Nigeria. J Bioremed Biodegrad 2017;8:1000393.
12. Nwokoye AI, Kuforiji OO, Oni PI. Studies on mycelial growth requirements of Pleurotus ostreatus (Fr.) Singer. Int J Basic Appl Sci 2010;10:47-53.
13. Kurtzman RH, Zadrazil F. Physiological and taxonomical considerations for cultivation of Pleurotus. Mushrooms 1998;16:299-343.
14. Anyakorah CI, Okafor N, Olatunji O. Carbon and nitrogen requirements for the cultivation of oyster mushroom Pleurotus sajor-caju. Niger Food J 2004;22:127-32.
15. Pokhrel CP, Kalyan N, Budathoki U, Yadav RK. Cultivation of Pleurotus sajor-caju using different agricultural residues. Int J Agric Pol Res 2013;1:19-23.
16. Ogbodo EN. Effect of crop residue on soil chemical properties and rice yield on an ultisol at Abakaliki, Southern Nigeria. World J Agric Sci 2011;7:13-8.
17. Abrishamkesh S, Gorji M, Asadi H, Bagheri GH, Purbabaee AA. Effects of rice husk biochar application on the properties of alkaline soil and lentil growth. Plant Soil Environ 2015;61:475-82.
18. Rollon RJ, Almendras FA, Ferraren DO. Effects of biochar application on potting media chemical properties, arbuscular mycorrhizal fungi spore density, growth and nutrient uptake of sorghum (Sorghum vulgare L.). AAB Bioflux 2017;9:135-99.
19. Moonmoon M, Uddin N, Ahmed S, Shelly NJ, Khan A. Cultivation of different strains of king oyster mushroom (Pleurotus eryngii) on saw dust and rice straw in Bangladesh. Saudi J Biol Sci 2010;17:341-5.
20. Mondal SR, Rehana MJ, Noman MS, Adhikary SK. Comparative study on growth and yield performance of oyster mushroom (Pleurotus florida) on different substrates. J Bangladesh Agric Univ 2010;8:213-20.
21. Adebayo GJ, Omolara BN, Toyin AE. Evaluation of yield of Oyster mushroom (Pleurotus pulmonarius) grown on cotton waste and cassava peel. Afr J Biotechnol 2009;8:215-8.
22. Frimpong-Manso J, Obodai M, Dzomeku M, Apertorgbor MM. Influences of rice husk on biological efficiency and nutrient content of Pleurotus ostreatus (Jacq. ex. Fr,)) Kummer. Int Food Res J 2011;18:249-54.
23. Salmones D, Mata SD, Waliszewski KN. Comparative culturing of Pleurotus spp. on coffee pulp and wheat straw:Biomass production and substrate biodegradation. Bioresour Technol 2005;96:537-44.
24. Atopia R, Obodai M, Pappoe J, Gidisu C, Takli RK. Cultivation of the oyster mushroom (Pleurotus ostraetus) on cellulosic residues from rice husk. Food Res Inst 2011;16:1-10.
25. Okwulehie IC, Okwujiako IA, Edeoga HO. Proximate, macro-element and vitamin composition of the fruit bodies of Pleurotus ostreatus var. Florida Eger. Grown on different substrates and substrate-supplementations. Food 2008;2:184-8.
26. Okwulehie IC, Odunze EI. Evaluation of the nutritional value of some tropical edible mushrooms. J Sustain Agric Environ Sci 2004a;6:157-62.
27. Okwulehie IC, Okorie DO, Okorie EG. Myco-chemical, proximate, mineral and vitamin composition of the pileus and stipe of two natural growing mushrooms found in Nigeria. Niger J Bot 2010;23:335-42.
28. Okwulehie IC, Nwosu CP, Okoroafor CJ. Pharmaceutical and nutritional prospects of two wild macro-fungi found in Nig. J Biotechnol 2007;6:567-72.
29. Patil SS. Productivity and proximate content of Pleurotus sajor-caju. Biosci Discov 2013;4:169-72.
30. Okwulehie IC, Okwuowulu EO, Ezeh CG, Ikechukwu GC. Yield potentials, nutritional and mycochemical properties of fruit-bodies of Pleurotus ostreatus var. Florida, grown on Andropogon gayanus straw;supplemented with Anthonotha macrophylla. Int J Mod Biol Res 2017;5:24-31.
31. Okwu DE. Phytochemical and vitamins contents of indigenous species of South Eastern Nigeria. J Sustain Agric Environ Sci 2004;6:163-70.
32. Onyeizu UR, Nwoko MC, Chukunda FA, Ukoima HN. Productivity, vitamins and heavy metals analysis of Pleurotus ostreatus (Jacq:Fri.) kumm. Fruit bodies cultivated on wood logs. Int J Inf Res Rev 2017;4:3890-4.
33. Okwulehie IC, Okwujako IA. The use of local Nigerian substrates for the production of Pleurotus ostraetua var Florida (Eger) sporophores. Glob Sci 2008;2:38-40.
34. Harbone JB. Phytochemical Methods. London:Champman and Hall Ltd.;1973. 11-13.
35. Steel RG, Torie JH. Principle and Procedures of Statistics. New York, USA:McGraw Hill Co Inc.;1980.
36. Okoi AI, Iboh CI. The effects of different substrates on sporophore yield, mineral and nutrient composition of Pleurotus tuber-regium Fries singer in Calabar, Nigeria. Int J Agric Sci Res 2015;4:126-31.
37. Rip S. Substrate formula's biological efficiency. Rip Snort mushroom cultivation catalog. 2010. 34.
38. Patil SS, Kadam RM, Shinde SL, Deshmukh SA. Effect of different substrate on productivity and proximate composition of Pleorotus floridanus. Int J Plant Sci 2008;3:151-3.
39. Obodai M, Sawyerr LC, Johnson PN. Yield of seven strains of oyster on sawdust using various woods for effective cultivation of oyster mushroom. Pak J Bot 2012;44:399-402.
40. Adejoye OD, Fasidi IO. Biodegradation of agro-wastes by some Nigerian white rot fungi. Bioresource 2009;4:816-24.
41. Hilang MT, Ferraro E. Phenolic Compounds in Food and their Effects on Health in Antioxidants and Cancer Prevention. Vol. 507. American Chemical Society (ACS). Symposium Series;1992. 8-12.
42. Edeoga HO, Eriata DO. Alaloids, tanins and saponin content of some medicinal plants J Med Aromat Plant Sci 2001;23:344-9.
43. Haslam E. Plant polyphenols (vegetable tannins) as drug possible of action. J Nat Prod 1996;59:205-15.