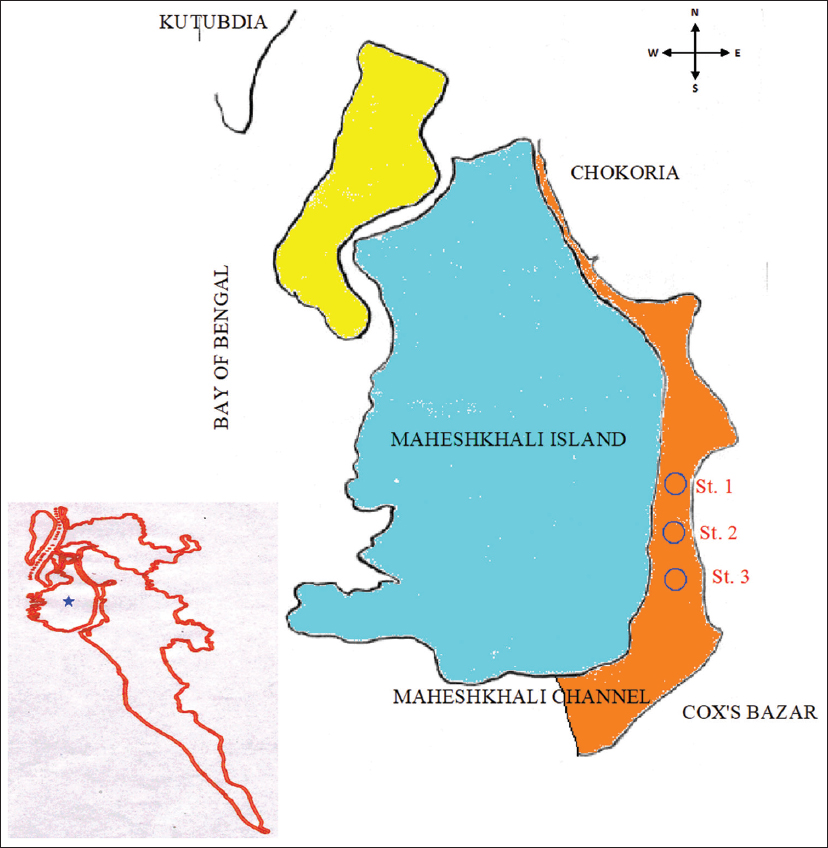
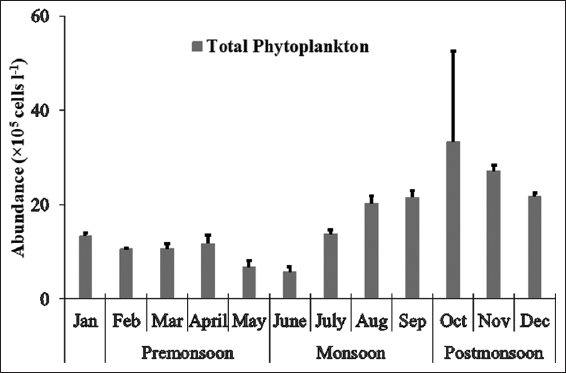
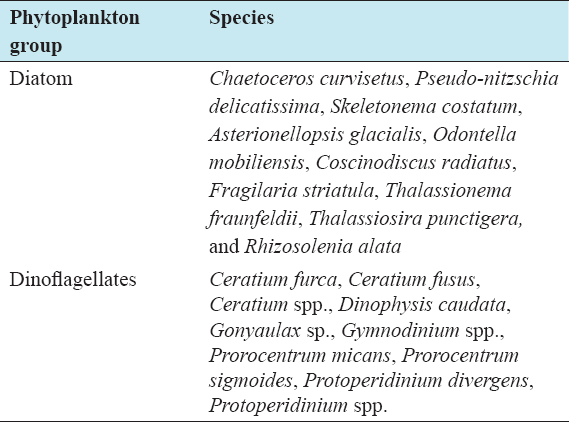
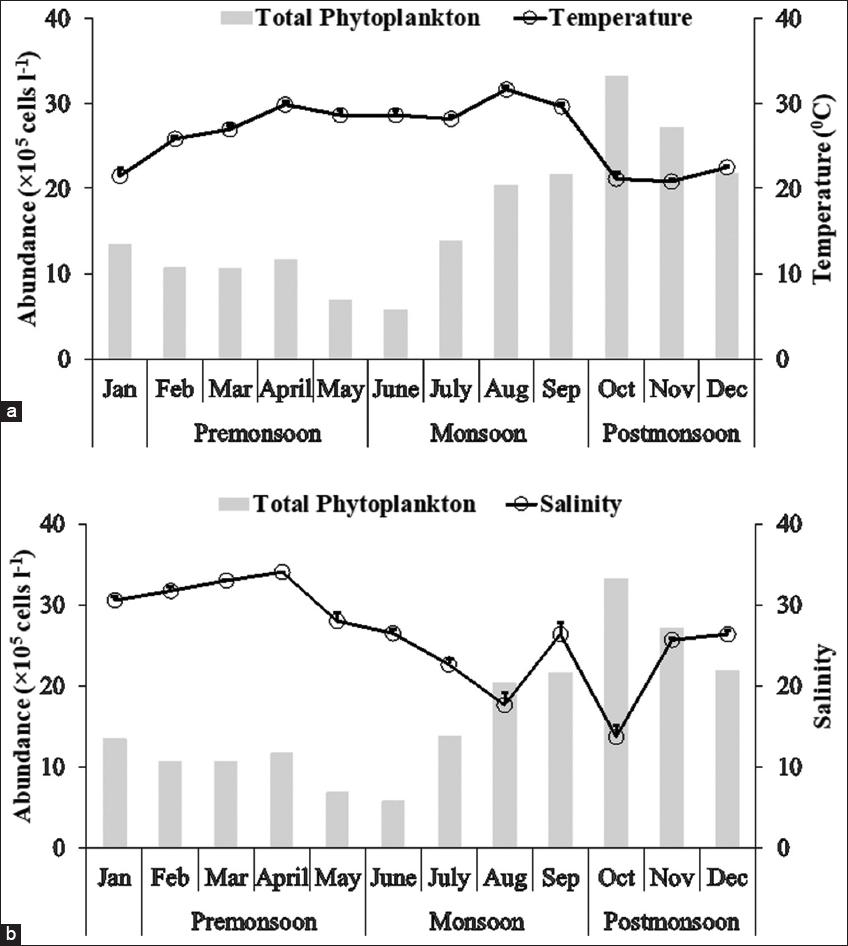
1. Nixon SW. Coastal marine eutrophication:A definition, social causes, and future concerns. Ophelia 1995;41:199-219.
2. Cloern JE. Our envolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 2001;210:223-53.
3. Rabalais NN. Eutrophication. In:Robinson AR, McCarthy J, Rothschild BJ, editor. The Global Coastal Ocean:Multiscale Interdisciplinary Processes, the Sea. Vol. 13. Ch. 21. Cambridge, MA:Harvard University Press;2004. 819-65.
4. Gordon AL, Giulivi CF, Takahashi T, Sutherland S, Morrison J, Olson D. Bay of Bengal nutrient-rich benthic layer. Deep Sea Res II 2002;49:1411-21.
5. Mukhopadhayay SK, Biswas H, De TK, Jana TK. Fluxes of nutrients from the tropical river hoogly at the land-ocean boundary of sundarbans, NE coast of Bay of Bengal, India. J Mar Syst 2006;62:9-21.
6. Godhe A, Karunasagar I, Karunasagar I. Gymnodinium catenatum on West cost of India. Harmful Algal News 1996;15:1.
7. Iyer CS, Robin RS, Sreekal MS, Kumar SS. Karenia mikimotoi bloom in Arabian sea. Harmful Algal News, 2008;37:9-10.
8. Karunasagar I, Gowda HS, Subburaj M, Venugopal MN, Karunasagar I. Outbreak of paralytic shellfish poisoning in Mangalore, West coast of India. Curr Sci 1984;53:247-9.
9. Karunasagar I, Joseph B, Philipose KK, Karunasagar I. Another outbreak of PSP in India. Harmful Algae News 1998;17:1.
10. de Sousa SN, Dileepkumar M, Sardessai S, Sarma VV, Shirodkar PV. Seasonal variability in oxygen and nutrients in the central and eastern Arabian sea. Curr Sci 1996;71:847-51.
11. Kumar SP, Murleedharan PM, Prasad TG, Gauns M, Ramaiah N, de Souza SN, et al. Why is the Bay of Bengal less productivity during summer monsoon compared to the Arabian sea?Geophys Res Lett 2002;29:2235.
12. D'Silva MS, Anil AC, Naik RK, D'Costa PM. Algal blooms:A perspective from the coasts of India. Nat Hazards 2012;63:1225-53.
13. Qasim SZ. Biological productivity of the Indian Ocean. Indian J Mar Sci 1977;6:122-37.
14. Radhakrishna K. Primary productivity of the Bay of Bengal during March-April 1975. Indian J Mar Sci 1978;7:58-60.
15. Gomes HR, Goes IJ, Siano T. Influence of physical processes and freshwater discharge on the seasonality of phytoplankton regime in the Bay of Bengal. Cont Shelf Res 2000;20:313-30.
16. Haque MM, Hossain MA, Khan S. Harmful Algal Blooms Associated with Mass Mortality of Fishes in the Bay of Bengal, Bangladesh. Florida, USA:10th International Conference of Harmful Algae;2002.
17. Hossain MS, Lin CK. Land Use Zoning for Integrated Coastal Zone Management:Remote Sensing, GIS and RRA approach in Cox's Bazar coast, Bangladesh. Bangkok, Thailand:ITCZM Publication Series, No.3, Asian Institute of Technology;2001.
18. Jewel MA, Khan S, Haque MM. Seasonal dynamics in the occurrence and abundance of Pseudo-nitzschia species in the Maheshkhali channel of the Bay of Bengal, Bangladesh. Bangladesh J Fish Res 2005;9:169-74.
19. Newell GE, Newell RC. Marine Plankton. London:Hutchinson and Co. Ltd.;1977.
20. Taylor FJ, Fukuyo Y, Larsen J. Taxonomy of harmful dinoflagellates. In:Hallegraeff GM, Anderson DM, Cembella AD, editors. Manual on Harmful Marine Microalge. IOC Manuals and Guides No. 33. Paris:UNESCO;1995. 283-309.
21. Steidinger KA, Tangen K. Dinoflagellate. In:Tomas CR, editor. Identifying Marine Phytoplankton. San Diego:Academic Press;1997.
22. Sanilkumar MG, Thomas AM, Philip AA, Hatha M, Sanjeevan VN, Saramma AV. First report of Protoperidinium bloom from Indian waters. Harmful Algal News 2009;39:15.
23. O'Herald. NIO Discovers Toxic Algal off Goa. Goa:O'Herald Newspaper;2001.
24. Anantharaman P, Thirumaran G, Arumugam R, Kanna RR, Hemaltha A, Kannathasan A, et al. Monitoring of Noctiluca bloom in Mandapam and Keelakarai coastal waters, South-East coast of India. Recent Res Sci Tech 2010;2:51-8.
25. Gopakumar G, Sulochanan B, Venkatesan V. Bloom of Noctiluca scintillans (Maccartney) in Gulf of Mannar, Southeast coast of India. J Mar Biol Ass India 2009;55:75-80.
26. Jewel MA, Haque MM, Haq MS, Khan S. Seasonal dynamics of phytoplankton in relation to environmental factors in the Maheshkhali channel, Cox's Bazar, Bangladesh. Bangladesh J Fish Res 2002;6:173-81.
27. Kay S, Casesar J, Janes T. Marine dynamics and productivity in the Bay of Bengal. In:Nicholls R, Hutton C, Adger W, Hanson S, Rahman M, Salehin M, editors. Ecosystem Services for Well-being in Delta. Cham:Palgrave Macmillan;2018. 263-75.
28. Shenoi SS, Shankar D, Shetye SR. Differences in heat budgets of the near-surface Arabian Sea and Bay of Bengal:Implications for the summer monsoon. J Geophy Res 2002;107:3052.
29. Bricker SB, Clement CG, Pirhalla DE, Orlando SP, Farrow DT. National Estuarine Eutrophication Assessment:Effects of Nutrient Enrichment in the Nation's Estuaries. National Oceanic and Atmospheric Administration, National Ocean Service, Special Projects Office and the National Centers for Coastal Ocean Service. Maryland:Silver Spring;1999.
30. Bricker S, Longstaff B, Dennison W, Jones A, Boicourt K, Wicks C, et al. Effects of nutrient enrichment in the nation's estuaries:A decade of change. National estuarine eutrophication assessment update. In:NOAA Coastal Ocean Program Decision Analysis Series 26 National Centers for Coastal Ocean Science. Maryland:Silver Spring;2007.
31. Whitall D, Bricker S, Ferreira J, Nobre AM, Simas T, Silva M. Assessment of eutrophication in estuaries:Pressure-state-response and nitrogen source apportionment. Environ Manage 2007;40:678-90.
32. Patil JS, Anil AC. Temporal variation of diatom benthic propagules in a monsoon influenced tropical estuary. Cont Shelf Res 2008;28:2404-16.
33. Kooistra WH, Gersonde R, Medlin LK, Mann DG. The origin and evolution of the diatoms:Their adaptation to a planktonic existence. In:Falkowski PG, Knoll AH, editors. Evolution of Primary Producers in the Sea. Amsterdam:Elsevier, Academic Press:2007. 207-49.
34. Kumar SP, Narvekar J, Nuncio M, Kumar A, Ramaiah N, Sardesai S, et al. Is the biological productivity in the Bay of Bengal light limited?Curr Sci 2010;98:1331-9.
35. Baek SH, Shimode S, Kikuchi T. Reproductive ecology of dominant dinoflagellate, Ceratium furca in the coastal area of Sagami Bay. Coastal Mar Sci 2006;30:344-52.
36. Bake SH, Shimode S, Kikichi T. Growth of dinoflagellate, Ceratium furca and Ceratium fusus in Sagami Bay, Japan:The role of temperature, light intensity and photoperiod. Harmful Algae 2008;7:163-73.
37. Latz MI, Jeong HJ. Effect of red tide dinoflagellate diet and cannibalism on the bioluminescence of the heterotrophic dinoflagellates Protoperidinium spp. Mar Ecol Prog Ser 1996;132:275-85.
38. Patil JS. Diatoms in benthic and pelagic environment. In:Studies on Ecology of Diatom. PhD Thesis. Goa:University of Goa;2003. 47-50.
39. Paul JT, Ramaiah N, Sardessai S. Nutrient regimes and their effect on distribution of phytoplankton in the bay of Bengal. Mar Environ Res 2008;66:337-44.
40. Rivera-Monroy VH, Madden CJ, Day JJ, Twilley RR, Vera-Herrera F, Alvarez-Guillen H. Seasonal coupling of a tropical mangrove forest and an estuarine water column:Enhancement of aquatic primary productivity. Hydrobiologia 1998;379:41-53.
41. Devassy VP, Bhattathiri PM, Qasim SZ. Trichodesmium phenomenon. Indian J Mar Sci 1978;7:168-86.