Study of blue-green algae in rural fish ponds, Mymensingh, Bangladesh
Saleha Khan1*, Roksana Jahan2, M. Aminur Rahman3, Md. Abdullah An Nur1, Md. Mahfuzul Haque1
1Department of Fisheries Management, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh, 2Department of Marine Fisheries and Oceanography, Sher-e-Bangla Agricultural University, Dhaka-1207, Bangladesh, 3Department of Fisheries and Marine Bioscience, Jashore University of Science and Technology, Jashore-7408, Bangladesh
ABSTRACT
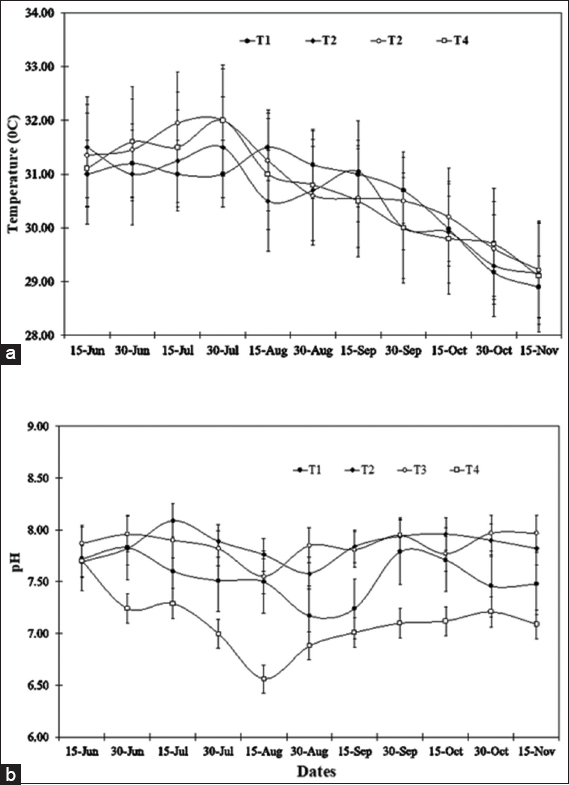
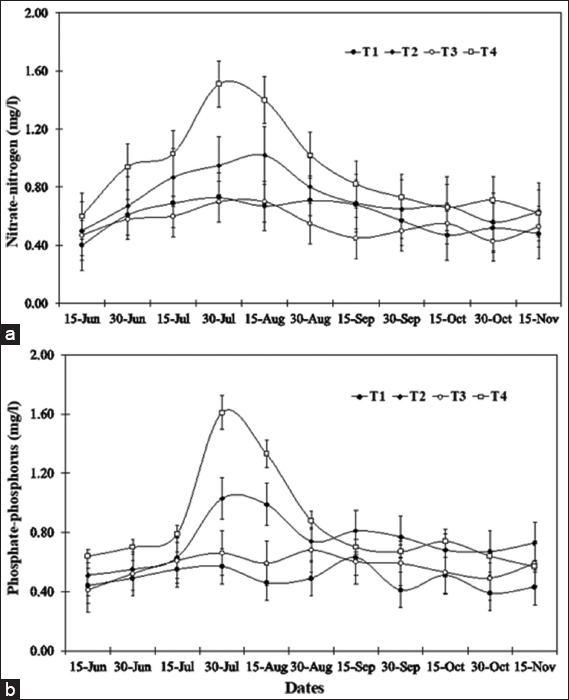
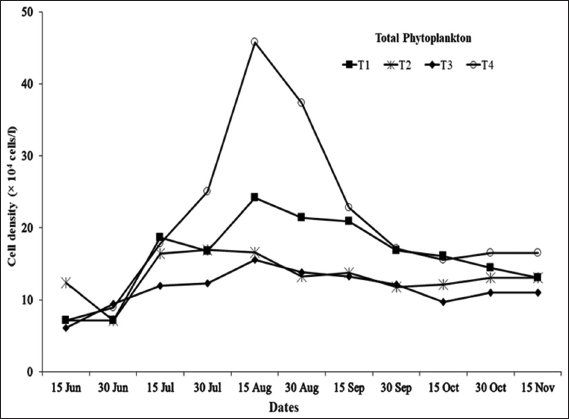
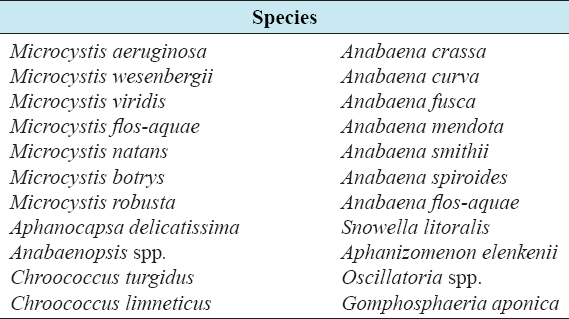
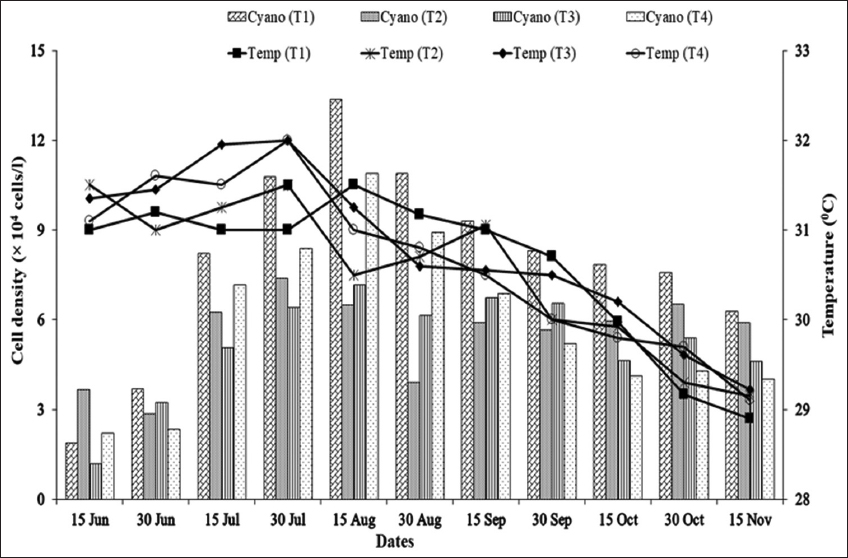
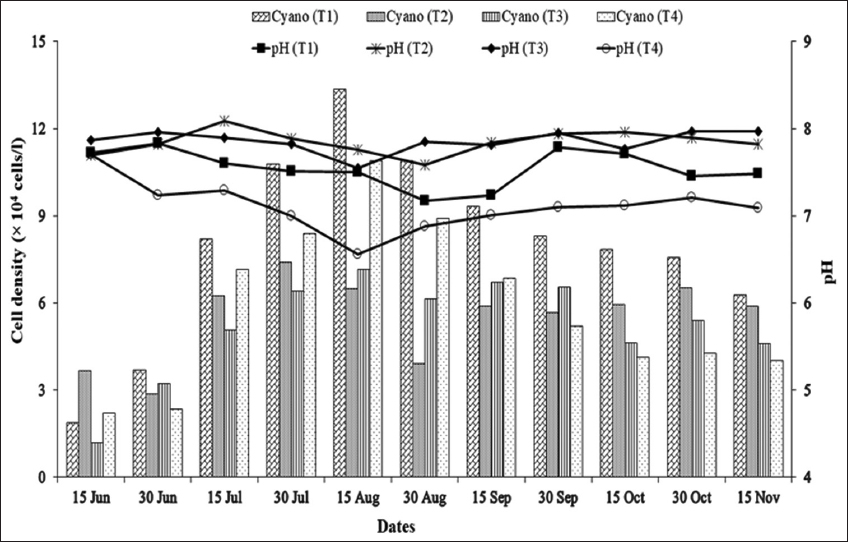
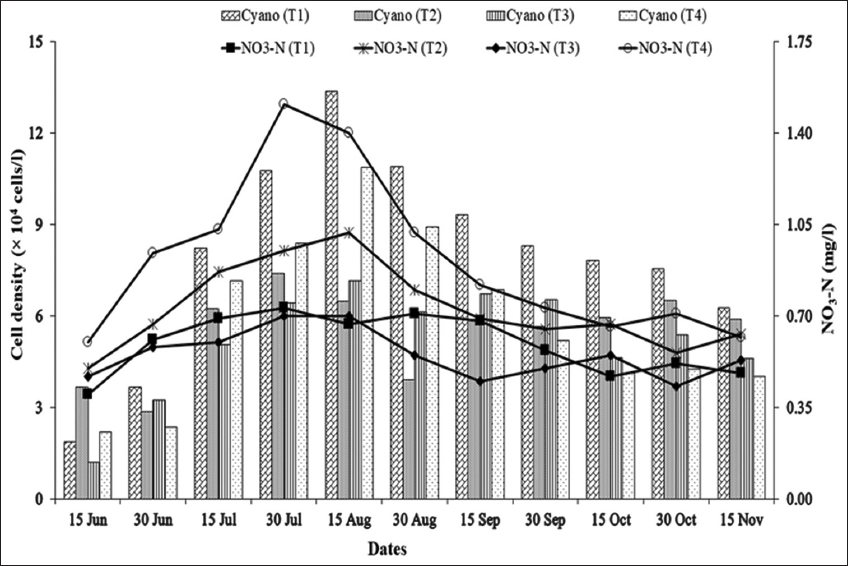
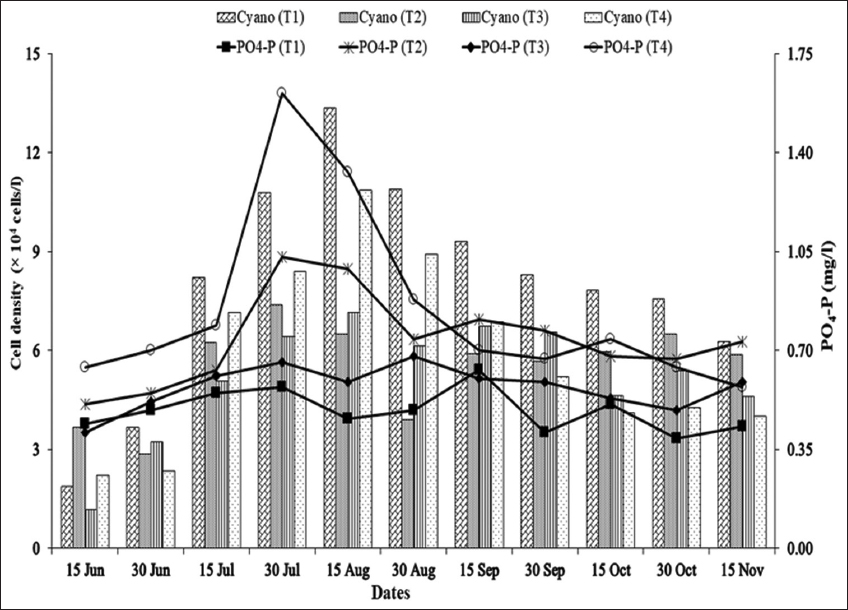
As blue-green algal blooms are very frequent in rural fish ponds in Bangladesh throughout the year, a study was carried out in 12 rural fish ponds under four treatments at Sutiakhali, Mymensingh, Bangladesh to see the relationship between environmental parameters and noxious Cyanobacteria. In ponds of treatment 1 (T1), one-third of the water surface was covered by duckweed (Lemna minor); in treatment 2 (T2), 0.5 kg lime/decimal/month was applied; in treatment 3 (T3), both duckweed (as in T1) and lime (as in T2) were applied; and in treatment 4 (T4), ponds were considered as control where none of duckweed or lime was applied. Around 51 genera of phytoplankton belonging to Euglenophyceae (3), Cyanophyceae (22), Cholorophyceae (16), and Bacillariophyceae (10) were recorded. Among Cyanobacteria, Microcystis spp., Anabaena spp., and Aphanizomenon spp. abundantly occurred throughout the study period. The highest cell density of Cyanobacteria was found in the ponds of T1 followed by T4, T2, and T3. Acidic pH (>6 and <7) was found to be most conducive for the bloom of cyanophytes. During the early bloom of cyanophytes, both nitrate-nitrogen and phosphate-phosphorus of the pond water were found high and there was a decreasing trend of nutrient concentration from the peak bloom period. In the ponds of T3, bloom of Cyanobacteria did not occur possibly due to alkaline pH and shade, and also nutrient absorption by duckweed. From the research findings, it can be concluded that the combined use of duckweed and lime can be effective in water quality maintenance as well as in controlling cyanobacteria blooms for sustainable fish production.
Keywords: Algal pollution, Cyanobacteria, nitrate-nitrogen, pH, phosphate-phosphorus