INTRODUCTION
Herbal drugs have gained importance and popularity in recent years because of their safety, efficacy and cost-effectiveness. The association of medical plants with other plants in their habitat also influences their medicinal values in some cases.[1] One of the important and well-documented uses of plant products is their use as hepatoprotective agents. Hence, there is an ever increasing need for safe hepatoprotective agent.[2] A number of herbs are traditionally used in different countries during drug or toxin-induced in hepatic, renal and cardiac disorders. Grewia umbellifera (GU) and Gmelina arborea (GA) leaves are commonly known as Phalasa and Kumizh is reported in Ayurvedic Pharmacopoeia. Traditionally, GU and GA are used for its cardioprotective, cardiotonic, diuretic, and aphrodisiac activities, and as an antidote to certain poisons.[3] Modern pharmacological studies have shown that leaves of GU and GA possess various positive effects in preventing various conditions including pain and inflammation, has diuretic activities, and is also effective in hyperlipidemic condition, cardiotoxicity, and stress. GU and GA are traditionally used for its cardioprotective, cardiotonic, general tonic, diuretic, aphrodisiac, antidote to certain poisons and scorpion stings, alternative purgative, and cooling effects.[4] It cures pain, ulcer, and fever and is used for pectoral-cough, asthma, and other bronchial disorders.[5] Hence, the present investigation was undertaken to evaluate the in vitro cytotoxicity of ethanol extract of GA and GU in HepG2 and Vero cell lines.
MATERIALS AND METHODS
Chemicals
3-(4,5-dimethylthiazol-2-yl)-5-diphenyltetrazolium bromide (MTT), fetal bovine serum (FBS), phosphate buffered saline (PBS), Dulbecco’s modified Eagle’s medium (DMEM), and trypsin were obtained from Sigma-Aldrich Co., St. Louis, USA. Ethylenediaminetetraacetic acid (EDTA), glucose and antibiotics from Hi-Media Laboratories Ltd., Mumbai, dimethyl sulfoxide (DMSO) and propanol from E. Merck Ltd., Mumbai, India.
Cell Lines and Culture Medium
Human, liver hepatocellular cells (HepG2) cell line and Vero cell lines were procured from National Centre for Cell Sciences, Pune, India. Stock cells were cultured in DMEM supplemented with 10% inactivated FBS, penicillin (100 IU/ml), streptomycin (100 µg/ml), and amphotericin B (5 µg/ml) in an humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with trypsin phosphate versene glucose solution (0.2% trypsin, 0.02% EDTA, and 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks, and all experiments were carried out in 96 microtiter plates (Tarsons India Pvt. Ltd., Kolkata, India).
Collection and Identification of Plant Material
The barks of GA were collected from south India, Kanyakumari district during the month of January and February. The plant was identified by S. Balasubramanium, ABS Botanical Garden, Salem.
Extract Preparation
The freshly collected barks were dried in the shade, then coarsely powdered. For extraction of crude phytochemical, 25 g of powdered bark material was kept in a closed conical flask with 20 mL various solvents such as petroleum ether, benzene, chloroform, ethanol, acetone, ethyl acetate, and distilled water in a shaker at room temperature for 24 h. After incubation, the extracts were filtered, and the extracts were collected and stored in the refrigerator at 4°C for further studies.
Preparation of Test Solutions
For cytotoxicity studies, each weighed test drugs were separately dissolved in distilled DMSO and volume was made up with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 1 mg/ml concentration and sterilized by filtration. Serial dilutions were prepared from this for carrying out cytotoxic studies.
Cytotoxicity Assay
The MTT assay was performed as described by Cardile et al.[6] The viability of the cell was assessed by MTT assay, which is based on the reduction of MTT by the mitochondrial dehydrogenase of intact cells to a purple formazan product. Cells were seeded in 96-well microplates (1 × 104 cells/well in 180 µl medium) and routinely cultured in a humidified incubator at 37°C in 5% CO2 for 24 h. The extracts were added in serial concentrations such as 125, 250, 500, and 1000 µg/ml and reincubated for 24 h. Then, the medium was discarded, and 30 µl of MTT dye solution (5 mg/ml in PBS) was added to every well and reincubated for 4 h. After removing un-transformed MTT reagent, 100 µl of DMSO was added to dissolve the formed formazan crystals. Amount of formazan was determined by measuring the absorbance at 540 nm using an enzyme-linked immunosorbent assay plate reader.
Light Microscopic Studies
Light microscopic examination of the cells was performed to observe the morphological changes after the treatment with ethanolic extracts of GA and GU for 24 h. HepG2 and Vero cells were grown in 35 mm sterile Petri plates and treated with ethanolic extracts of GA and GU at the concentration of 125, 250, 500, and 1000 µg/ml for 24 h. The cells were then fixed for 5 min with 10% methanol, PBS. The morphological changes were observed under inverted microscope (Nikon, Japan).
Statistical Analysis
Values are expressed as mean ± standard deviation. The results were statistically evaluated using independent sample–t-test using SPSS 10.0 student version. P < 0.05 was considered statistical significant.
RESULTS
Effect of Ethanolic Extracts of GA and GU on the Cytotoxicity of HepG2 and Vero Cells
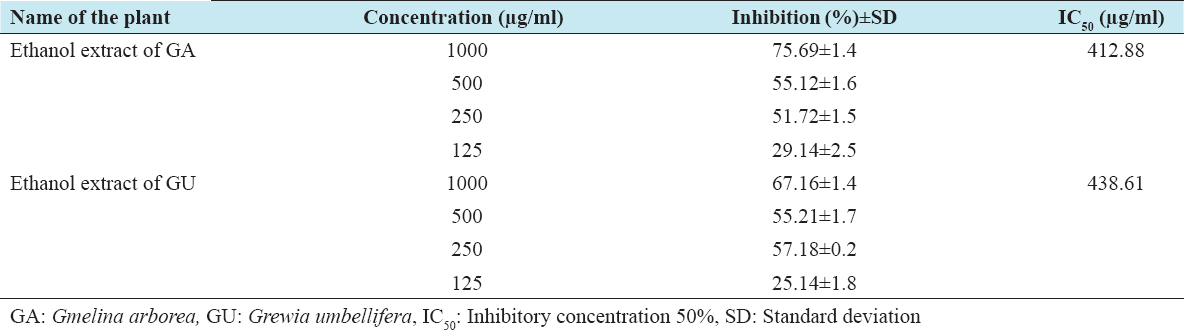
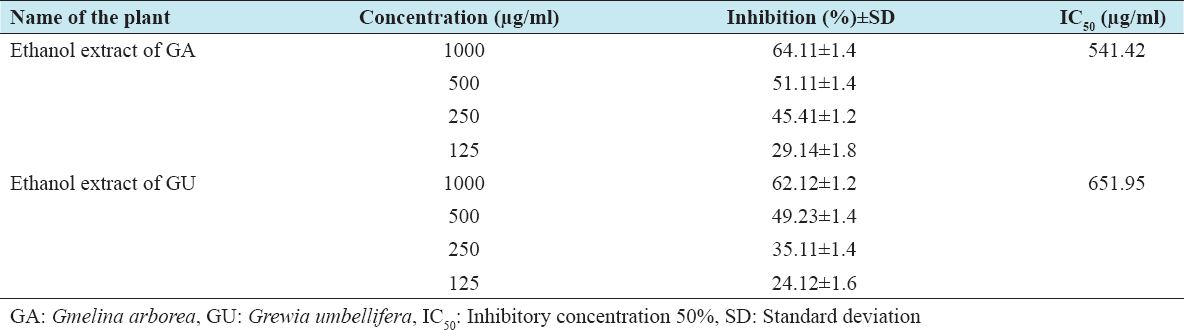
The cytotoxic effects of ethanolic extracts of GA and GU on HepG2 and Vero cells were performed by the MTT method, which is reliable to detect proliferation of cells. The results of the MTT assay are shown in Tables 1 and 2. The results clearly confirm that the exposure of ethanolic extracts of GA and GU at different concentrations such as 125, 250, 500, and 1000 µg/ml for 24 h resulted in decrease of cell proliferation in a dose-dependent manner. The percentage of inhibitory concentration 50% (IC50) inhibition of cell proliferation was found to be initiated at the concentration of 412.88 µg/ml of ethanolic extracts of GA and GU in HepG2 cells and 438.61 µg/ml in Vero cells, respectively.
Table 1: Cytotoxic properties of test drugs against HepG2 cell line of GA and GU
Table 2: Cytotoxic properties of test drugs against Vero cell line of GA and GU
Effect of Ethanolic Extracts of GA and GU on the Apoptosis and Morphology of HepG2 and Vero Cells
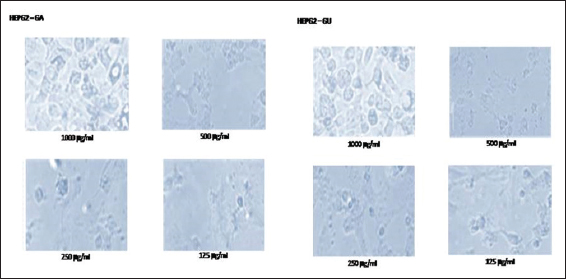
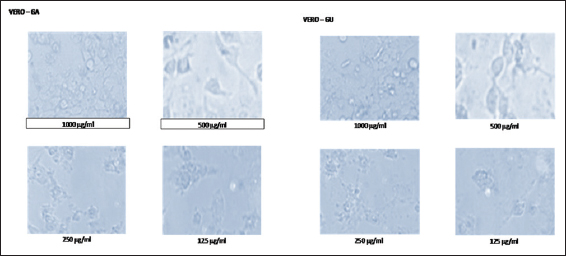
In this study, the light microscopic analysis was studied to determine the presence of apoptosis in ethanolic extracts of GA and GU treated HepG2 and Vero cells. The morphological changes of control and ethanolic extracts of GA and GU treated HepG2 and Vero cells at the concentration of 125, 250, 500, and 1000 µg/ml after 24 h of exposure are shown in Figures 1 and 2. The HepG2 and Vero cells treated with dose-dependent concentrations of ethanolic extracts of GA and GU significantly changed the structural alterations and reduction cells populations.
Figure 1: Effect of ethanolic extracts of Gmelina arborea and Grewia umbellifera on the apoptosis and morphology of HepG2 cells
Figure 2: Effect of ethanolic extracts of Gmelina arborea and Grewia umbellifera on the apoptosis and morphology of vero cells
DISCUSSION
The biological activity of any phytocompounds depends on the type of chemical composition and the concentration of active constituents as well as types and developmental stages of the cancer.[7] The screening of plants for their anticancer properties used cell-based assays and established cell lines, in which the cytotoxic effects of plant extracts could be measured. MTT assay is a nonradioactive, fast and economical assay widely used to quantify cell viability and proliferation. MTT is a yellow water-soluble tetrazolium salt. Metabolically active cells are able to convert the dye to water-insoluble dark blue formazan by reductive cleavage of the tetrazolium ring.[8] The result of our study revealed that ethanol extract of GA and GU has a cytotoxic effect on human liver hepatocellular cells (HepG2) cell line in a concentration-dependent manner. The extract showed moderate therapeutic values HepG2 cell line with IC50 values 412.88 and 438.61, respectively. Morphological studies also confirmed that the ethanol extract of GA and GU has got the potential cytotoxic effect. The result of our study revealed that ethanol extract of GA and GU has a cytotoxic effect on Vero cell line in a concentration-dependent manner. The extract showed moderate therapeutic values on Vero cell line with IC50 values 541.42 and 651.95, respectively. Morphological studies also confirmed that the ethanol extract of GA and GU has got the potential cytotoxic effect.