INTRODUCTION
Colon cancer remains a serious health problem around the world despite its advances in diagnosis and treatment.[1] It ranks third in overall cancer incidence and is the second most common cause of cancer-related mortality in the United States.[2] The prognosis is especially poor for metastatic colorectal cancer (CRC), with overall 5-year survival rates of 5-8%.[3] The incidence rate continues to rise as people change their lifestyles and food habits.[4] Diets high in red meat and processed food show established risk factors for CRC.[5] In this connection, various enzymes have been reported in the correlation between colon cancer incidence and their alterations in the biochemical levels. Na+/K+, Ca2+, Mg2+ adenosine triphosphatase (ATPase) is a membrane bound enzyme involved in the transport of various cations and the steroidal glycoside. Inhibition of this enzyme results in a depletion of intracellular Mg2+ and Ca2+ that leads to the pathogenesis of the various disorders. The transport takes place against ion gradient and the energy required for the process is provided by ATP, which is hydrolyzed to ADP and Pi during these transport phenomena.
In cancer condition, the levels of Na+/K+, Ca2+, Mg2+ ATPase were seen decreased and this may be due to the inhibition of the phosphorylation of ATPase by the chemical carcinogens. Experimental colon cancer induced by 1, 2-dimethylhydrazine (DMH) in rats a potent carcinogen that acts as a DNA methylating agent-inducing colon tumors in experimental animals. It is further transported to colon through bile or blood to generate its ultimate carcinogenic metabolite, diazonium ion which elicits an oxidative stress by methylating biomolecules of colonic epithelial cells, and leads to promutagenic events as a result of inflammation and tumor promotion. The inflammatory pathway between liver and liver-mediated DMH induction in colon, serves a channel, and knocks off the immune system during its metastatic approach. Tumor markers comprise predominately the substances that are produced by malignant cells or the substances that are produced by other cells under the influence of malignant cells that are determined in body fluids.[6] Tumor markers can be either newly synthesized substances or the substances that can be found in normal organisms in much lower concentrations.[7] The determination of tumor markers in cancer condition reveals the extent of tumor pathogenesis and its metastatic approach to distinct organs. In the present study, the levels of tumor markers such as carcinoembryonic antigen (CEA) and CA 19-9 were found increased and this may be due to the cytotoxic degradation of normal cells and its malignant transformation tendency to release excess levels of the marker enzymes from the tumor cells.
Cytokines are small glycoproteins produced by a number of cell types predominantly leukocytes, which regulate immunity, inflammation, and hematopoiesis.[8] Hence, recent studies have focused on the regulation of inflammation and biochemical enzyme alterations in cancer studies. Natural chemo-preventive drugs have been analyzed in respect to its less side effects and anti-inflammatory properties. Myrtenal and essential oil of monoterpene family present in cumin, pepper, mint, eucalyptus, etc., was found to suppress the proliferation of murine B16 melanoma, human HL-60 leukemia cells, and other carcinogenic process. In this connection, various studies have postulated its biological activities such as anti-malarial, anti-plasmodial, anti-radicular, hypocholesterolemic, gonadotrophic, cyclooxygenase-inhibitor, and immunostimulant effects.[9] A variety of studies have been followed, but there is a paucity of information regarding its anti-inflammatory property; hence, the present study determines the anti-inflammatory property of myrtenal in DMH-induced colon cancer in experimental rats.
MATERIALS AND METHODS
Reagents
DMH, myrtenal was purchased from Sigma Chemical Company, St. Louis, MO, USA. All the other chemicals used in this study were of analytical grade available Commercially.
Experimental Animals
Experiments were carried out with 5-week-old male Wistar rats procured from the Central Animal House Facility, Dr. A. L. M. Postgraduate Institute of Basic Medical Sciences, University of Madras, Taramani, Chennai - 600 113. They were maintained in the controlled environment conditions of temperature and humidity on alternative 12 h light/dark cycle, noise level maintained below 85 dB and had unrestricted access to standard diet consisting of 24% protein, 4.5% fat, and 4% fiber. The experiment was sanctioned and approved by the Institutional Animal Ethical Committee (IAEC No. 01/13/2013).
Experimental Design
The experimental animals were divided into four groups, each group comprising six animals.
-
Group I: Control animals fed with standard diet and pure drinking water
-
Group II: Animals were administered with 20 mg/kg body weight (b.wt.) of DMH, in 1 mM ethylenediaminetetraacetic acid (EDTA), pH adjusted to 6.5 with 1 mM NaOH and subcutaneously injected once in a week for 15 weeks
-
Group III: Animals were treated with myrtenal (230 mg/kg b.wt.) with corn oil as vehicle for 30 weeks by intragastric administration. Myrtenal treatment was started 1 week prior to the first dose of 20 mg/kg b.wt. of DMH (as in Group II)and continued till end of the experimental period
-
Group IV: Animals were treated with myrtenal (230 mg/kg b.wt.) for 30 weeks by intragastric administration to assess the cytotoxicity if any, induced by myrtenal, and rats were referred as drug control.
After the end of the experimental period, the rats were fasted overnight and anesthetized using diethyl ether and sacrificed. A portion of the colon was used for histopathological studies and remaining tissue was homogenized in 0.1 M Tris-HCl buffer pH 7.4 and centrifuged, the supernatant was used for biochemical studies.
Colon Analysis
Colons were excised from experimental rats, and were blotted dry and opened longitudinally, with the inner surface examined for visible macroscopic lesions. Tumor weight was determined for the colons. Immediately following sacrifice, colons were removed and washed with ice-cold saline, and colon homogenates (10%) were prepared in ice-cold Tris-buffered saline (Tris 50 mM and NaCl 150 mM; pH 7.2) then centrifuged at 10,000 g for 10 min at 4°C and were stored as aliquots at or below −20°C for subsequent assays.
Estimation of Membrane Bound ATPases
ATPases catalyzes the conversion of ATP into adenosine diphosphate. During the conversion, one molecule of inorganic phosphate is liberated. The inorganic phosphate is estimated according to the method of Fiske and Subbarow.[10] The proteins were precipitated with trichloroacetic acid. The free filtrate reacts with acid molybdate to form phosphor-molybdic acid which is reduced by the addition of 1-amino 2-naphthol-4-sulphonic acid to produce blue color. The intensity of the color is proportional to the amount of inorganic phosphate present in the sample.
Preparation of Hemolysate and Isolation of Erythrocyte Membrane
Blood collected with EDTA was centrifuged at 2000 rpm for 20 min at 4°C. The packed cells were washed with isotonic saline to remove the buffy coat. An aliquot of packed cells were then washed three times with isotonic Tris-HCl (0.3 M, pH 7.4) buffer. An aliquot of 1.0 ml washed cells were lysed using 9.0 ml of hypotonic Tris-HCl buffer (0.015 M, pH 7.2). The lysed cells were centrifuged for 30 min at 15,000 rpm. The pellet was repeatedly washed with hypotonic Tris-HCl buffer until a clear pale pink or colorless supernatant was obtained. The resulting erythrocyte membrane pellet was suspended in 0.01 M Tris-HCl buffer, pH 7.4 for subsequent analysis.
Na+ K+ - ATPase was assayed according to the method of Israel et al.[11] Ca2+ - ATPase was estimated as described by the method of Hjertén and Pan.[12] Mg2+ - ATPase was assayed by the method of Ohnishi.[13]
Tumor Markers
Estimation of CEA
The UBI MAGIWEL CEA Quantitative CM-201 is a solid phase enzyme-linked immunsorbent assay kit. This test provides quantitative measurement of CEA in serum.
Estimation of CA 19-9
The UBI MAGIWEL CA 19-9 Quantitative CM-701 is a solid phase enzyme-linked immunsorbent assay kit. This test provides quantitative measurement of in serum CA 19-9.
Inflammatory Markers
Determination of cytokines
The levels of cytokines such as interleukins (IL-6; IL-10; IL-17) in serum were determined using specific enzyme-linked immunosorbent assay kits (Biosource, California, US). The analyses were performed according to instructions of the manufacturer’s. Standard plots were constructed using standard cytokines and the concentrations for unknown samples were calculated from the standard plot.
RESULTS
Effect of Myrtenal on Membrane Bound ATPase
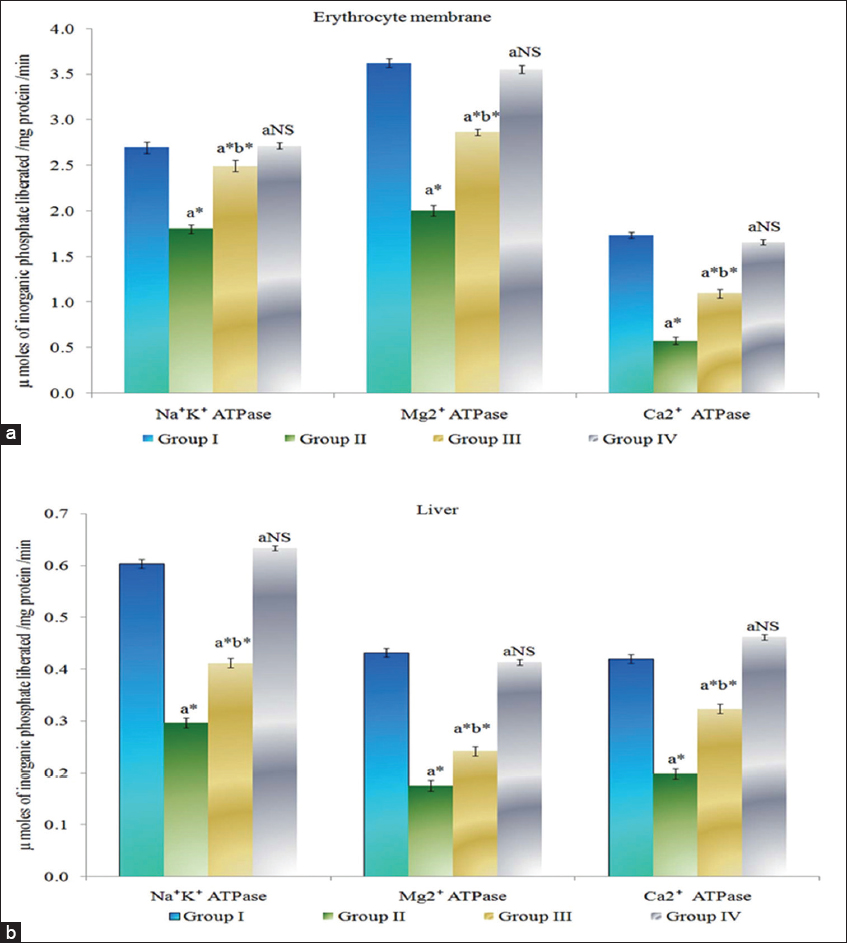
The activities of Na+/K+, Ca2+, Mg2+ ATPase in erythrocyte membrane and liver of control and experimental animals are provided in Figure 1. The membrane bound ATPase were found to be significantly decreased (P < 0.05) in erythrocyte membrane and liver of Group II colon cancer-bearing animals compared to Group I control animals. Myrtenal-treated Group III animals show a significant increase (P < 0.05) in the levels of membrane bound ATPase in erythrocyte membrane and liver compared to Group II cancer bearing animals. Group IV myrtenal alone treated animals show no significant change compared to Group I control animals.
Figure 1: (a and b) Effect of myrtenal on adenosine triphosphatase levels in erythrocyte membrane and liver tissue of control and experimental animals. Results are expressed as mean ± standard deviation for six animals, (a) Group II, III, and IV compared with Group I, (b) Group III compared with Group II. NS – Nonsignificant, *P < 0.05
Effect of Myrtenal on Levels of Tumor Markers
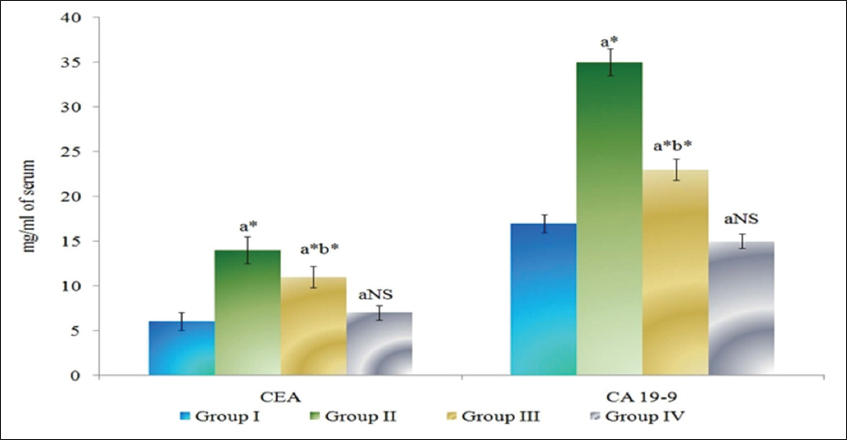
The levels of tumor markers CEA and CA 19-9 in serum of control and experimental animals are presented in Figure 2. Tumor marker enzymes levels were found to be significantly increased (P < 0.05) in DMH-induced Group II colon cancer-bearing animals compared to Group I control animals. The Group III myrtenal-treated animals show a significant decrease (P < 0.05) in the levels of tumor markers compared to Group II animals. No significant changes were observed in Group IV myrtenal alone treated animals compared to Group I control animals.
Figure 2: Effect of myrtenal on levels of tumor markers in serum of control and experimental animals. Results are expressed as mean ± standard deviation for six animals, (a) Group II, III, and IV compared with Group I, (b) Group III compared with Group II. NS – Nonsignificant, *P < 0.05
Effect of Myrtenal on Levels of Cytokines
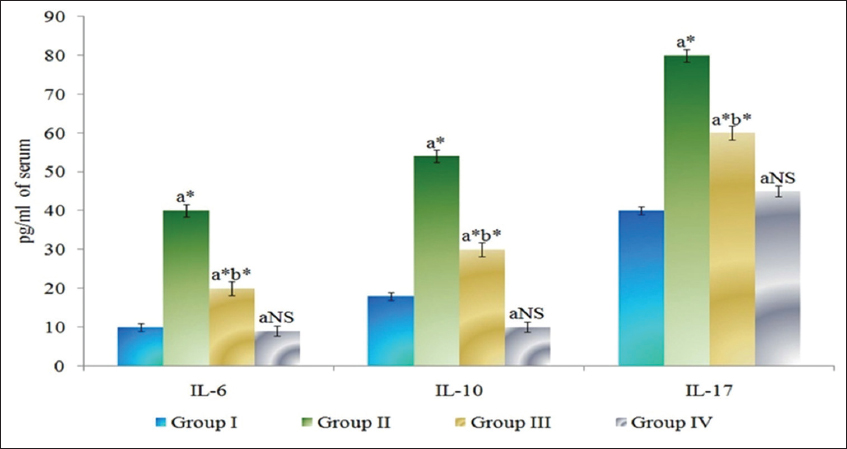
The levels of cytokines in serum of control and experimental animals are presented in Figure 3. IL-6, IL-10, and IL-17 were found to be significantly increased (P < 0.05) in DMH-induced Group II cancer-bearing animals compared to Group I control animals. The Group III myrtenal-treated animals show a significant decrease (P < 0.05) in the levels of cytokines compared to group II animals. No significant changes were observed in Group IV myrtenal alone treated animals compared to Group I control animals.
Figure 3: Effect of myrtenal on levels of cytokines in serum of control and experimental animals. Results are expressed as mean ± standard deviation for six animals, (a) Group II, III, and IV compared with Group I, (b) Group III compared with Group II. NS – Nonsignificant, *P < 0.05
DISCUSSION
The present study provides evidence for a synergy between inflammation and colon cancer prognosis. Chemical-induced experimental animals have expressed increased levels of tumor markers and inflammatory marker.[14] In this connection, the increase in the levels of membrane bound ATPase delivers effective cellular and electrogenic pump in normal cells.[15] In colon cancer, condition induced by DMH the anaerobic pathways of tumor cells express decreased ATP levels as this in turn reflect the production of membrane ATPases. Activation of vesicles in the cytoplasm is favored by Ca2+ ATPase, and thus, in cancer condition the altered levels of calcium ATPases results in inactivation of major cellular components. Calcium-modulated protein is a calcium-binding messenger protein that mediates many crucial processes such as inflammation, metabolism, apoptosis, smooth muscle contraction, intracellular movement, short-term and long-term memory, and the immune response.[16] Thereby, alterations in the levels of calcium ATPases regulate the normal cellular functions. Mg2+ ATPase functions as a key factor in the activation of cofactor to regulate kinase enzymes.[17] In colon cancer, condition the levels of Mg2+ ATPase inhibit as a cofactor activator. Myrtenal-treated animals expressed altered levels of membrane bound ATPase to near normal and this might be due its specific activation of cell membranes by preventing lipid peroxidation reaction.
Colon cancer cases are mostly proved by increased CEA levels in the experimental models.[18] In addition to the levels of tumor markers biopsy and other diagnosis are essential to confirm the extent of tumorogenesis. Tumor markers are also essential in the diagnosis of cancer treatment as they respond to the chemo-preventive drugs. In the present scenario, myrtenal-treated animals expressed very little tumor marker enzymes compared to colon cancer bearing animals and this might be due to the anti-proliferative effect of myrtenal against DMH-induced animals. In addition, cytokines have a much larger distribution of sources for their production, with nearly all cells that have a nucleus capable of producing IL-1, IL-6, and tumor necrosis factor alpha, particularly endothelial cells, epithelial cells, and resident macrophages.[19] The levels of cytokines such as IL-6, IL-10, and IL-17 were seen increased in DMH-induced animals and this might be due to severe inflammation on normal cells and their profound inflammatory action during cancer conversion. Myrtenal treatment reduced the levels of cytokines and their activity in the cellular environment and this might be due to the immunomodulating property of myrtenal that modulate the immune response across the whole body against chemical carcinogens and its pathological effect. In addition, regulated cytokine levels help in combat against various diseases including cancer. Hence, myrtenal supplementation might have triggered the beneficial cytokine modulation against cancer cells. Thereby, the present study provides an insight on the effect of myrtenal on inflammatory cytokines and their regulation which can regulate colon cancer proliferation.